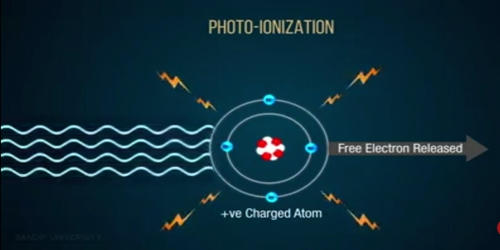
Photoionization is the physical process in which an ion is formed from the interaction of a photon with an atom or molecule. This ionization produced in a medium by the action of electromagnetic radiation. It is the interaction of electromagnetic radiation with matter resulting in the dissociation of that matter into electrically charged particles. It is the phenomenon in which the absorption of electromagnetic radiation by an atom in a gas induces the atom to emit a bound electron and thereby become ionized.
The photoionization technique ionizes molecules using a high-energy photon source, such as UV radiation, while the flame ionization technique burns organic molecules in a hydrogen flame. It is likely important in a wide range of XRB, CV), and the physics of astrophysical situations, including AGN, galactic binaries (BHT, photoabsorption is in every spectrum.
Cross-section
Not every interaction between a photon and an atom, or molecule, will result in photoionization. The probability of photoionization is related to the photoionization cross-section of the species, which depends on the energy of the photon (proportional to its wavenumber) and the species being considered. The simplest example, the photoelectric effect (q.v.), occurs when light shines on a piece of metal, causing the ejection of electrons.
The ionization of the molecules occurs when the UV energy imparted on the molecule exceeds its ionization energy/potential. The ionization of an atom or molecule by photons of electromagnetic radiation. A photon can only remove an electron if the photon energy exceeds the first ionization potential of the atom or molecule. In the case of molecules, the photoionization cross-section can be estimated by examination of Franck-Condon factors between a ground-state molecule and the target ion. The application of a potential across the cell containing the ionized molecules leads to an ion current that is measured.
For photon energies below the ionization threshold, the photoionization cross-section is near zero. But with the development of pulsed lasers, it has become possible to create extremely intense, coherent light where multi-photon ionization may occur. Ionization is a process by which electrically neutral atom or molecule is converted into an electrically charged atom or molecule called ions, under the impact of a high energy source. The methods of ion detection are the same in both these sensors. When an electric field gradient is applied across the ionization region to drive the ions to the electrodes, the ions release their charges to produce signals. At even higher intensities (around 1015 – 1016 W/cm2 of infrared or visible light), non-perturbative phenomena such as barrier suppression ionization and rescattering ionization are observed.