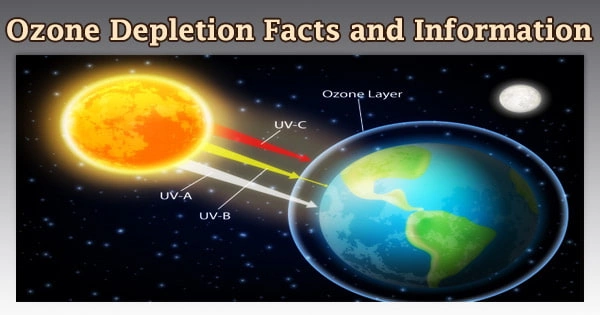
The ability of the ozone layer to absorb damaging UV radiation that might otherwise be destructive to people’s health is well known. Walking outside would be miserable without the ozone layer. Since the late 1970s, two related occurrences have been observed: a constant drop in the total amount of ozone in Earth’s atmosphere (the ozone layer) of around 4%, and a significantly bigger springtime decrease in stratospheric ozone around Earth’s polar regions.
The thinning is mainly noticeable over Antarctica and in the polar regions. Ozone depletion is a major environmental issue because it increases the amount of ultraviolet (UV) radiation reaching the Earth’s surface, which causes skin cancer, cataracts, and genetic and immune system damage.
Produced chemicals, particularly manufactured halocarbon refrigerants, solvents, propellants, and foam-blowing agents (chlorofluorocarbons (CFCs), HCFCs, halons), are the principal causes of ozone depletion and the ozone hole (ODS). The Montreal Protocol, which was ratified in 1987, was the first of numerous extensive international accords implemented to reduce ozone-depleting chemical production and use.
The ozone layer is projected to recover over time as a result of continuing worldwide cooperation on this subject. Ozone is composed of three linked oxygens that rise to the stratosphere and absorb a significant quantity of UV light, safeguarding all life below.
However, “ozone-depleting substances” (or ODS) comprised mostly of chlorine and bromine make their way to the stratosphere, where they deplete the oxygen in the O3 and render it incapable of absorbing UV light. Humans have made progress in preventing harm to the ozone layer during the last 30 years by limiting the use of certain substances.
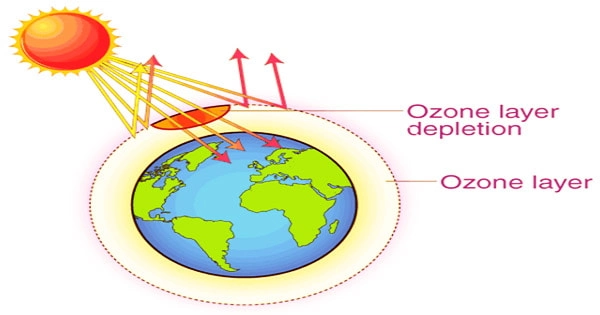
However, there is still work to be done to maintain and restore the stratosphere, which is located 9 to 18 miles (15 to 30 kilometers) above the Earth’s surface. The ozone hole and ozone depletion have sparked widespread concern about increasing cancer risks and other negative consequences.
Since before the 1980s, human activities have had a considerable impact on the global concentration and distribution of stratospheric ozone. Furthermore, scientists have reported that from at least 1980, major annual declines in average ozone concentrations began to occur.
The ozone layer blocks harmful ultraviolet (UVB) light wavelengths from entering the Earth’s atmosphere. Skin cancer, sunburn, lifelong blindness, and cataracts are all caused by these wavelengths, which are expected to rise rapidly as ozone levels drop, as well as damaging plants and animals.
Because of its protective role, atmospheric ozone is sometimes referred to as “good” ozone. This is not to be confused with tropospheric, or ground-level, “bad” ozone, which is a major component of air pollution and has been related to respiratory disease. By the mid-1990s, ozone levels had stabilized and were beginning to rebound in the 2000s, as the southern hemisphere’s jet stream was no longer flowing towards the south pole and may even be reversing.
The human effect is devastating for plants that are particularly susceptible to UV light, such as wheat and barley, which are two of humanity’s most important crops. The highest ozone reductions occurred at high latitudes (near the poles), while the smallest reductions occurred at lower latitudes (the tropics).
Furthermore, observations of the atmosphere suggest that the ozone layer’s depletion increased the quantity of UV radiation reaching the Earth’s surface. The recovery is likely to continue throughout the next century, with the ozone hole expected to close by 2075, returning to pre-1980 levels. NASA announced in 2019 that the ozone hole was the smallest it had ever been since it was discovered in 1982.
According to the US Environmental Protection Agency, one atom of chlorine can destroy more than 100,000 ozone molecules, destroying ozone considerably faster than it can be restored. Although most ozone-depleting compounds have been outlawed, the chemicals that have already been released can take up to 80 years to reach the upper atmosphere, so it will be some time before our protective barrier is fully functional again.
The lower stratosphere has seen the greatest significant drop in ozone. The Montreal Protocol on Compounds That Deplete the Ozone Layer was signed in 1987 as a landmark agreement to phase out CFCs and other ozone-depleting substances. It has been accepted by all 197 UN member countries.
Without the agreement, the United States would have experienced an additional 280 million cases of skin cancer, 1.5 million deaths from skin cancer, 45 million cataracts, and the world would have been at least 25% hotter. Theoretical studies indicating that chlorine and bromine generated from halocarbons in the stratosphere react with and destroy ozone have been confirmed by atmospheric measurements.
The majority of the ozone that is destroyed happens in the lower stratosphere, as opposed to the considerably lesser ozone depletion that occurs in the high stratosphere due to homogenous gas-phase interactions. The chlorine catalytic cycle, in which single chlorine atoms and related compounds steal single oxygen atoms from ozone molecules, was first presented as a possible explanation for the ozone decline.
Other possibilities evolved because more ozone loss occurred than could be explained by the amount of reactive chlorine available in the polar areas at the time through recognized processes. When it comes to dangerous gases produced by coolants, the world is still in the dark. Some transitional alternatives for hydrochlorofluorocarbons (HCFCs), which are less hazardous but still destructive to ozone, are still in use.