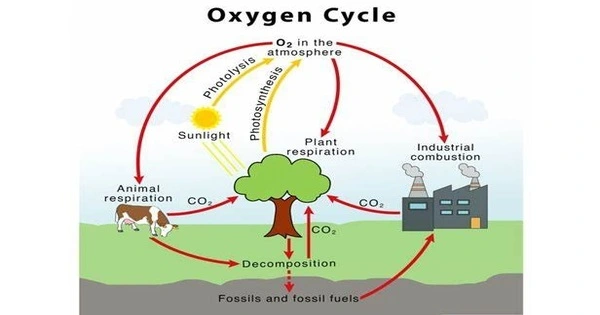
The oxygen cycle describes the transport of oxygen through the atmosphere (air), biosphere (plants and animals), and lithosphere (the Earth’s crust). The oxygen biogeochemical cycle is a set of processes that exchange oxygen between the Earth’s atmosphere, biosphere, lithosphere, and hydrosphere. The oxygen cycle shows how free oxygen is made accessible in each of these locations and how it is utilized. Oxygen is an essential element for life on Earth and plays an important part in many metabolic processes. The oxygen cycle’s key components are photosynthesis, respiration, and breakdown.
The oxygen cycle is the biogeochemical cycle of oxygen atoms moving between different oxidation states in ions, oxides, and molecules via redox processes inside and between Earth’s spheres/reservoirs. The term oxygen in the literature usually refers to the most frequent oxygen allotrope, elemental/diatomic oxygen (O2), because it is a common product or reactant in many biogeochemical redox processes throughout the cycle. The oxygen cycle processes are classified as biological or geological, and they are evaluated as either a source (O2 production) or a sink (O2 consumption).
Here’s an overview of the key processes involved in the oxygen cycle:
(1) Photosynthesis
The primary source of oxygen on Earth is photosynthesis, which occurs in green plants, algae, and some bacteria. During photosynthesis, these organisms use sunlight, carbon dioxide (CO2), and water to produce glucose and oxygen. The general formula for photosynthesis is:
6CO2 + 6H2O + light energy → C6H12O6 + 6O2
(2) Respiration
Organisms, including plants, animals, and microorganisms, engage in respiration to obtain energy from glucose produced during photosynthesis. In the process of respiration, oxygen is consumed, and carbon dioxide is released.The simplified formula for respiration is the reverse of photosynthesis:
C6H12O6 + 6O2 → 6CO2 + 6H2O+ energy
(3) Decomposition
Decomposition is the breakdown of dead organic matter by bacteria, fungi, and other decomposers. During decomposition, organic compounds are broken down into simpler substances, and oxygen is consumed in the process. The breakdown products may include carbon dioxide, water, and other compounds.
(4) Combustion:
Combustion is the process of burning, which releases energy by combining oxygen with a fuel source. This process is common in activities such as the burning of fossil fuels, wood, and other organic materials. Combustion releases carbon dioxide and water vapor, and oxygen from the atmosphere is consumed.
(5) Diffusion and Mixing:
Oxygen in the atmosphere is also subject to diffusion and mixing, as it moves between the atmosphere, oceans, and land surfaces.
Oxygen is one of the most abundant elements on Earth, accounting for a significant amount of each major reservoir. The silicate and oxide minerals of the crust and mantle contain the vast majority of Earth’s oxygen (99.5 percent by weight). The Earth’s atmosphere, hydrosphere, and biosphere collectively contain less than 0.05% of the total mass of oxygen. Aside from O2, additional oxygen atoms are found in diverse forms across the surface reservoirs in the molecules of biomass, H2O, CO2, HNO3, NO, NO2, CO, H2O2, O3, SO2, H2SO4, MgO, CaO, Al2O3, SiO2, and PO4.
The balance between these processes helps maintain a relatively stable concentration of oxygen in the Earth’s atmosphere, supporting the respiration of aerobic organisms. Human activities, such as deforestation and the burning of fossil fuels, can impact the oxygen cycle by altering the rates of photosynthesis and combustion, leading to changes in atmospheric oxygen levels.