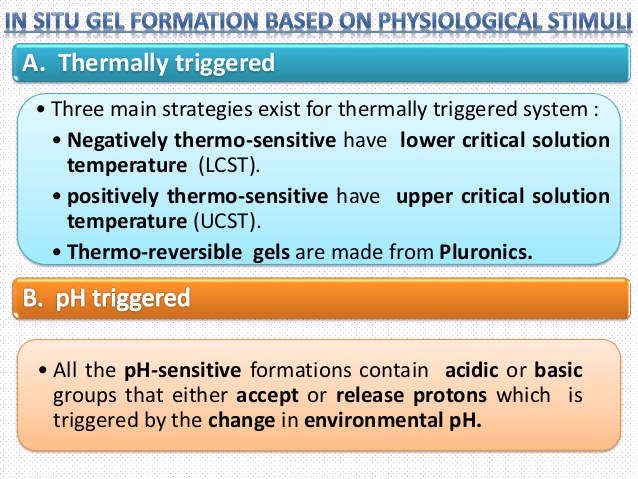
Negatively thermo sensitive hydrogels tend to shrink or collapse as the temperature is increased above the LCST, and swell upon lowering the temperature below the LCST. The change in the hydration state, which causes the volume phase transition, reflects competing hydrogen bonding properties, where inter-molecular hydrogen bonding of the polymer molecules are favoured compared to a solubilization by water. Thermodynamics can explain this with a balance between entropic effects due to the dissolution process itself and due to the ordered state of water molecules in the vicinity of the polymer.
Negatively Thermo Sensitive Hydrogels