Neuroscientists have identified a group of brain cells that influence mice’s motivation to perform tasks for rewards. Increasing the activity of the cells causes a mouse to work harder or more vigorously. The neurons include a feature that prevents the mouse from becoming addicted to the reward if it overdoes it. The findings suggest new therapeutic approaches for treating mental illnesses that impair motivation, such as depression.
The limbic system is a grouping of specific neural networking structures associated with emotional behaviors and memory. The limbic system is made up of four major brain regions: the limbic cortex, the hippocampal formation, the amygdala, and the hypothalamus. Among the limbic system structures, the amygdala is important in controlling motivational behaviors such as reward-related motivation, as well as appetitive and aversive behaviors. The nucleus accumbens, a brain structure located in the ventral striatum, serves as a functional link between the limbic and motor systems and is important in motivational behavior.
A lack of motivation is a symptom of depression. Professor Bo Li of Cold Spring Harbor Laboratory (CSHL) and CSHL Adjunct Professor Z. Josh Huang discovered a group of neurons in the mouse brain that influences the animal’s motivation to perform tasks for rewards. Up to a point, increasing the activity of these neurons causes a mouse to work faster or more vigorously. These neurons contain a feature that prevents the mouse from developing an addiction to the reward. The findings could lead to new therapeutic strategies for treating mental illnesses that affect motivation in humans, such as depression.
We discovered a group of neurons in the mouse brain that influences the animal’s motivation to perform tasks for rewards. Up to a point, increasing the activity of these neurons causes a mouse to work faster or more vigorously. These neurons contain a feature that prevents the mouse from developing an addiction to the reward. The findings could lead to new therapeutic strategies for treating mental illnesses that affect motivation in humans, such as depression.
Professor Bo Li
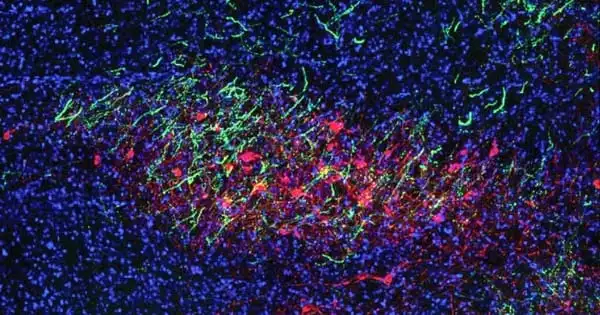
The anterior insular cortex is a brain region that is important for motivation. A group of neurons in this area that activates a gene called Fezf2 (Fezf2 neurons) are active when mice perform both physical and cognitive tasks. Li and his colleagues hypothesized that these neurons do not affect the mouse’s ability to complete the task, but rather influence the mouse’s motivational drive.
Mice were taught to lick the spout of a water bottle in order to receive a small sugar reward. Mice licked more vigorously when researchers increased the activity of these Fezf2 neurons. The mice would lick more slowly if the neuron activity was reduced. Another experiment in which mice ran on a wheel to receive a reward yielded a similar result, according to the researchers. When the Fezf2 neurons were stimulated, the mice ran faster. The same thing happened with other tasks.
Li and his colleagues were taken aback when they discovered a feature that prevents mice from becoming addicted to the tasks and their rewards. Even when mice were satiated after drinking their fill of sugar water, they did not lick or run faster to get more sugar, even when the researchers increased the activity of the Fezf2 neurons.
In terms of motivational states induced by rewarding or aversive stimuli, stimulation of amygdala glutamatergic neurons projecting to the VTA area induces aversive behavior, whereas stimulation of its GABAergic projections to the VTA area induces rewarding behavior.
Choosing one reward over another is important for reducing the possibility of addictive behaviors. Stimulation of neurons in the amygdala’s central nucleus in conjunction with receiving a specific reward has been shown to increase the magnitude of reward motivation while decreasing the range of reward selection. Stimulation of these neurons raises the amount of effort required to obtain that particular reward.
Depression, a mental state associated with a lack of motivation, is characterized primarily by an inability to enjoy pleasure. Individuals who suffer from depression have an overactive amygdala. Negative emotions such as grief, anxiety, frustration, and fear activate the amygdala, which in turn activates the hypothalamic-pituitary-adrenal axis and increases stress hormone production and secretion.
Finding a way to fine-tune the human equivalent of these neurons could benefit people suffering from mental illnesses such as depression. “We want to selectively increase the person’s motivation so that they can do the things that they need to do,” Li says, “but we don’t want to create addictive drugs.”