An interdisciplinary team presents a laser-driven technology for producing nanoparticles from materials such as copper, cobalt, and nickel oxides. Photoelectrodes are produced in this manner at the usual printing speed, for example, for a wide range of applications such as the generation of green hydrogen.
An interdisciplinary team from the Max Planck Institute of Colloids and Interfaces presents for the first time in the journal Nature Communications a laser-driven technology that allows them to create nanoparticles such as copper, cobalt, and nickel oxides. Photoelectrodes are produced in this manner at the usual printing speed, for example, for a wide range of applications such as the generation of green hydrogen.
Previous methods only produced such nanomaterials with a high energy input in traditional reaction vessels over a long period of time. The scientists can deposit small amounts of material on a surface while simultaneously performing chemical synthesis in a very short time using the laser’s high temperatures. ‘I knew I was onto something big when I discovered the nanocrystals under the electron microscope,’ says Junfang Zhang, the study’s first author and doctoral researcher. The discovery turned into a new and environmentally friendly method for synthesizing materials that can, among other things, efficiently convert solar energy into electricity.
An interdisciplinary team presents a laser-driven technology that enables them to create nanoparticles out of materials such as copper, cobalt and nickel oxides.
Without taking any detours with sunlight to get to hydrogen: ‘These days, the vast majority of green hydrogen is produced from water using electricity generated by solar panels and stored in batteries. ‘We can use solar light directly by using photoelectrodes,’ says Dr. Aleksandr Savateev.
The newly developed principle works with transition metal oxides, primarily copper, cobalt, and nickel oxides, which are all excellent catalysts. The unique property of these oxides is the variety of crystal forms (nanocrystals such as nanorods or nanostars), which influences their surface energy. Each structure has a unique impact on catalytic reactions. As a result, it is critical that these nanostructures be made targeted – or even untargeted but repeatable.
The developed technology could also be used to find new catalysts quickly and efficiently. ‘We can create different catalysts side by side by simply changing the composition and conditions, and then test them in parallel right away,’ says Dr. Felix Löffler, adding, ‘but now we need to work on making the catalyst systems more persistent in all applications.’
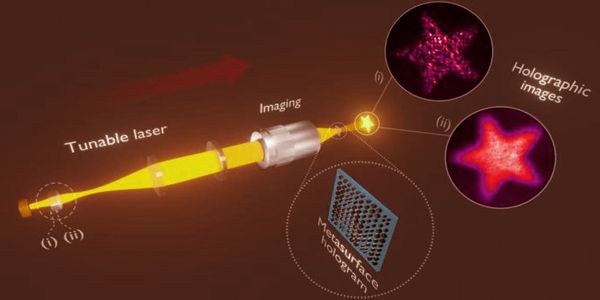
The method
Material is transferred from a donor to an acceptor carrier in a manner similar to that of a typewriter. The ‘ink’ on the former is a solid polymer mixed with metal salts, while the latter is a thin carbon nitride film on a conductive electrode. The salts are transferred to the acceptor along with the molten polymer by targeted laser irradiation. The high temperatures cause the salts to react in milliseconds, transforming them into metal oxide nanoparticles with the desired morphology.
Without detours with sunlight to hydrogen
‘Today, the majority of green hydrogen is generated from water using electricity generated by solar panels and stored in batteries. We can use solar light directly by using photoelectrodes,’ says Aleksandr Savateev. The newly developed principle works with transition metal oxides, primarily copper, cobalt, and nickel oxides, which are all excellent catalysts. The unique property of these oxides is the variety of crystal forms (nanocrystals such as nanorods or nanostars), which influences their surface energy. Each structure has a unique impact on catalytic reactions.
As a result, it is critical that these nanostructures be made targeted – or even untargeted but repeatable. The developed technology could also be used to find new catalysts quickly and efficiently. ‘We can create different catalysts side by side by simply varying the composition and conditions, and then test them in parallel right away,’ says Dr. Felix Löffler, adding, ‘but now we need to work on making the catalyst systems more persistent in all applications.’
Material is transferred from a donor to an acceptor carrier in a manner similar to that of a typewriter. The ‘ink’ on the former is a solid polymer mixed with metal salts, while the latter is a thin carbon nitride film on a conductive electrode. The salts are transferred to the acceptor along with the molten polymer by targeted laser irradiation. The high temperatures cause the salts to react in milliseconds, transforming them into metal oxide nanoparticles with the desired morphology.