Lithium-sulfur batteries have never fulfilled their promise as the next generation of renewable batteries for electric vehicles and other gadgets. But ?Donghai Wang, a mechanical engineer at SMU, and his research team discovered a means to extend the life of these Li-S batteries while also increasing their energy levels.
The researchers were able to prevent Li-S batteries from developing an undesired side effect known as polysulfide dissolution, which occurs over time and shortens their lifespan.
“This breakthrough could lead to more durable, long-lasting batteries,” stated Wang, the Brown Foundation Chair of Mechanical Engineering and Professor of Mechanical Engineering at SMU Lyle.His research focuses on the design and synthesis of nanostructured functional materials and energy storage technologies like Li-ion batteries and also beyond Li-ion technology.
Our cathode uses multiple sulfur bonding tethers, atomic adsorption, and fast Li-ion/electron transport at the molecular level. This combination allows for real-time re-bonding and adsorption of any unbound sulfur species, thus effectively eliminating soluble polysulfides and extending the battery’s cycle life.
Donghai Wang
A study published in the journal Nature Sustainability shows that the team’s newly developed hybrid polymer network cathode allows Li-S batteries to deliver over 900 mAh/g (milliampere-hours per gram mass), compared to the typical 150-250 mAh/g capacity in lithium-ion batteries. That means it has a much higher amount of electrical energy it can preserve.
“It also offers excellent cycling stability – outperforming conventional lithium-sulfur batteries,” Wang said.
Cycling capacity measures the number of times a battery can charge and discharge before its capacity degrades sharply. A higher cycling capacity means a longer-lasting battery.
Assisting Wang with designing the cathode were researchers from Pennsylvania State University, Pacific Northwest National Laboratory, Brookhaven National Laboratory, University of Illinois at Chicago and the Argonne National Laboratory.

A cost-effective solution that delivers more energy
What makes Li-S batteries so promising as a source of renewable energy is that they’re more cost-effective and can hold more energy than traditional ion-based rechargeable batteries. But there is a key problem with these batteries.
“Over the years, the battery community has struggled to mitigate the negative effects of polysulfide dissolution,” Wang said.
All batteries have a positive terminal and a negative terminal. Inside the battery, the chemical reaction that is continuously happening between these two terminals provides power or electricity to the battery.
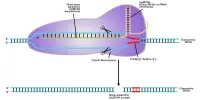
In Li-S batteries, a positive electrode or terminal containing sulfur is known as a cathode, while a negative electrode made of lithium metal is known as an anode. The electrolyte, or the substance that allows ions to move between the battery’s two ends, is sandwiched between these components. Sulfur, however, is far from suitable for use as an electrode.
When lithium ions connect with sulfur atoms at the cathode, they produce soluble polysulfide molecules that wander into the electrolyte, degrading the cathode and diminishing the battery’s ability to withstand numerous charging cycles. This is referred to as polysulfide dissolution. Wang and his team solved this problem by creating a hybrid polymer network cathode.
“Our cathode uses multiple sulfur bonding tethers, atomic adsorption, and fast Li-ion/electron transport at the molecular level,” Wang explained. “This combination allows for real-time re-bonding and adsorption of any unbound sulfur species, thus effectively eliminating soluble polysulfides and extending the battery’s cycle life.”