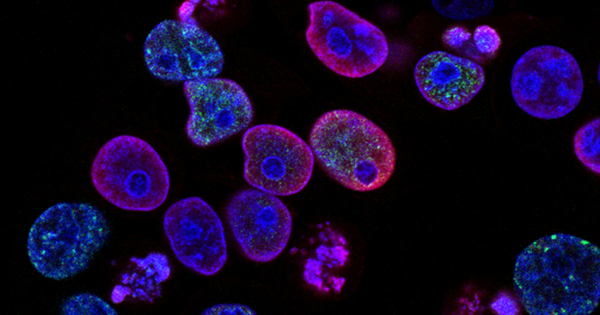
Cancer cells undergo metabolic changes that allow them to continue their rapid growth and proliferation. One of the most important metabolic characteristics of cancer cells is their preference for glucose as a fuel source. They exhibit the Warburg effect, in which they heavily rely on glycolysis, a process that converts glucose into energy even in the presence of oxygen, rather than oxidative phosphorylation, the more efficient energy production pathway used by normal cells.
A new nutrient source for pancreatic cancer cells has been discovered by researchers. Uridine is a molecule that provides insight into both biochemical processes and potential therapeutic pathways. The findings show that cancer cells can adapt when they lack glucose.
A new nutrient source for pancreatic cancer cells has been discovered by researchers at the University of Michigan Rogel Cancer Center. Uridine is a molecule that provides insight into both biochemical processes and potential therapeutic pathways.
The findings, published in Nature, demonstrate that cancer cells can adapt when they are deprived of glucose. Other nutrients that serve as fuel sources for pancreatic cancer have previously been identified by researchers; this study adds uridine to the list.
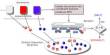
The findings revealed that the enzyme uridine phoshorylase-1, or UPP1, metabolizes uridine. Blocking UPP1 had a significant impact on the growth of pancreatic tumors in mice, indicating the importance of testing uridine-blocking drugs as potential new treatment options.
Costas Lyssiotis
Pancreatic tumors have few functional blood vessels and thus cannot easily access nutrients from the bloodstream, such as glucose. Costas Lyssiotis, Ph.D., Maisel Research Professor of Oncology and study lead investigator, explained that cancer cells become hungry when they lack the proper nutrients. “We know they’re still growing, obviously, but what are they growing with?” he asked. “These findings indicate that, under certain conditions, uridine is one of those fuels.”
When asked about the study’s significance, Zeribe Nwosu, Ph.D., one of the study’s co-first authors, says, “The ability of cancer to switch to alternative nutrients has fascinated me for a long time.” Blocking such compensatory switches may lead to new treatments, which is what we hope this study will do.”
Uridine is present in the tumor microenvironment, but its exact source, and how cancer cells access it, remains a mystery. “Part of the picture is it’s in the bloodstream, but we don’t know where it’s coming from specifically,” said Lyssiotis. “Likely, it’s coming from multiple places, and so far we haven’t been able to pin it to a single source.”

Events referred to by Lyssiotis as “times of crisis” – when cells do not have enough nutrients due to limited blood access and/or intense competition between cells — could provide insight into why and where cells turn to uridine. “The cancer cells seem to be sensing the concentrations of glucose and uridine in the local environment to inform their adaptation,” says Matt Ward, another co-first author. This unknown regulatory process, as well as a cancer-promoting mutation in the KRAS gene, which is common in pancreatic cancer, are identified by Lyssiotis’ team as two ways that cancer cells control their uridine usage.
Lyssiotis and his colleagues in the Sadanandam lab at the Institute for Cancer Research in London have been working on this project for nearly a decade. They used a technology that tests hundreds of different nutrients to see which ones promote the growth of pancreatic cancer. Normally, researchers look at common nutrients such as sugar, protein, and fat, but Lyssiotis’ team took a different approach. “We used a large panel with over 20 pancreatic cell lines and around 200 different nutrients to assess the different ways pancreatic cancer cells grow,” he said. “What exactly do they metabolize?” We discovered uridine as a result of this method.”
This method also provides therapeutic insight. The findings revealed that the enzyme uridine phoshorylase-1, or UPP1, metabolizes uridine. Blocking UPP1 had a significant impact on the growth of pancreatic tumors in mice, indicating the importance of testing uridine-blocking drugs as potential new treatment options.
“With new drug targets and new therapeutic approaches, there is potential to better understand and treat pancreatic cancer,” said Sadanandam, a co-author on the study.