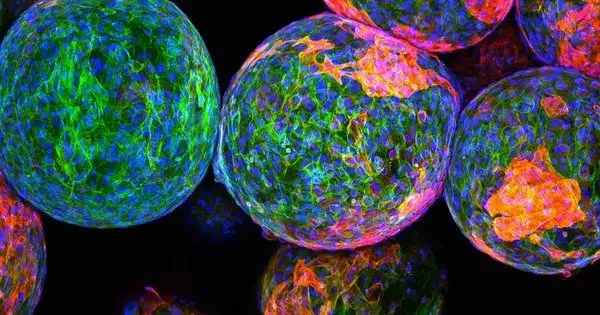
Immune rejection of biomedical implants has been a major challenge in medical device and tissue engineering research. When foreign materials enter the body, such as implants or prosthetic devices, the immune system can perceive them as dangerous and initiate an immunological response to remove or destroy these materials. This can result in inflammation, implant failure, and a variety of other consequences.
A team from the University of Arizona College of Medicine – Tucson found a protein that appears to help drive this response in order to understand more about what causes the body to reject biomedical implants. They hope their discoveries will improve the design and safety of biomedical implants. The findings were published in the journal Nature Biomedical Engineering.
Breast implants, pacemakers, and orthopedic hardware have revolutionized medicine, but a considerable number of them are rejected by the body and must be removed. The culprit is a poorly understood immune response known as foreign body response (FBR), in which the body surrounds the implant in scar tissue.
The proposed approach, according to senior author Geoffrey Gurtner, MD, FACS, department chair of surgery, and co-lead author Kellen Chen, Ph.D., assistant research professor of surgery, is a departure from the conventional thought process for addressing implant failure, which has thus far relied on using so-called biocompatible materials that are better tolerated by the body but do not completely eliminate the risk of FBR.
We targeted RAC2 along with other pathways and observed a significant reduction in the level of FBR – up to three-fold. We think targeting these pathways could serve as a potential therapy to mitigate or even prevent clinically significant FBR in humans.
Dharshan Sivaraj
To learn why some immune systems form thick capsules around implants while others do not, the researchers collected capsule samples from 20 patients who had their breast implants removed: 10 with severe reactions and 10 with mild reactions. In samples taken from patients with severe responses, a protein known as RAC2 was shown to be substantially expressed.
“When we examined the severe fibrotic samples, RAC2 was one of the most important proteins we found,” Chen stated. “Because it seemed to drive a lot of downstream pathways, we decided to explore a little more closely.”
Immune cells activate RAC2 and other proteins in response to mechanical stress induced by the implants, which call additional immune cells, including those that can unite to assault a huge intruder.
“That foreign body, which is very stiff and causes stress on the external environment, activates these immune cells to aggregate to that area,” Chen explained. “They start merging with each other, making massive cells that spit out fibrous proteins like collagen and other products.”
To confirm RAC2’s role in FBR, the team blocked the expression of RAC2 in animal models.
“We targeted RAC2 along with other pathways and observed a significant reduction in the level of FBR – up to three-fold,” said Dharshan Sivaraj, a research fellow in the Department of Surgery and co-lead author of the study. “We think targeting these pathways could serve as a potential therapy to mitigate or even prevent clinically significant FBR in humans.”
Because RAC2 is exclusive to immune cells, a treatment that inhibits it may theoretically solely influence immune cells while having no effect on other cells in the body. Gurtner and Chen, who are also members of the UArizona Cancer Center, say the next step is to create a more specific version of this drug ready for human use, and their team is already working with the Arizona Center for Drug Discovery at the R. Ken Coit College of Pharmacy to develop it.
“We believe that local targeted therapy is better. Maybe there are ways to conjugate this drug onto an implant with some sort of coating to minimize systemic problems,” Gurtner said.
Tech Launch Arizona, the University of Arizona’s technology commercialization office, is working with the team to translate the innovation from the laboratory to the marketplace, where they hope it will have a real-world impact on patients and their healthcare providers.
The Gurtner Lab conducted this study in collaboration with teams at Stanford University School of Medicine, the University of Texas Southwestern Medical Center, and the University Hospital Regensburg in Germany. Other College of Medicine – Tucson Department of Surgery authors included Hudson C. Kussie, research professional; Katharina Fischer, MD, postdoctoral fellow; and Artem A. Trotsyuk, PhD, assistant research professor.
“Establishing a complete understanding of the molecular mechanisms driving the foreign body response presents the final frontier in developing truly bio-integrative medical devices,” Gurtner said.
The causes of FBR are not well understood. The prevailing theory, according to Chen, is that FBR is a reaction to the chemical composition of the implant. In contrast, this study shows that the implant causes stress areas in the body, resulting in an excessive immune response.
“The cells interact with this new material in the same way that I would with something soft versus something hard.” Touching a table activates mechanical circuits in my fingertips, indicating that it is harder than a cushion. Similarly, as the cells interact with the implant and surrounding tissue, they become activated as a result of the increased mechanical stress,” Chen explained. “The immune cells realize there is a foreign body there, and they react by building a fibrotic capsule that surrounds the implant in an attempt to shield it off.”