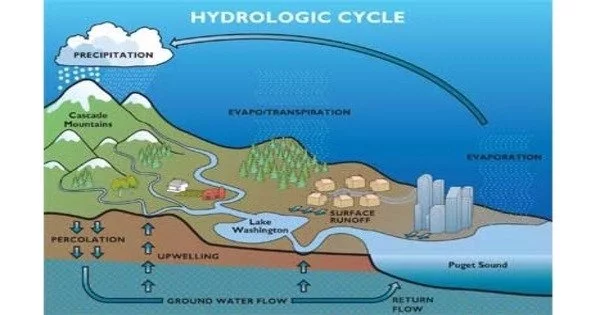
The hydrogen cycle is the circulation of hydrogen atoms through various forms in the environment. Hydrogen is the most abundant element in the universe, but it is usually found in molecular form as H2. In the environment, hydrogen is constantly cycled between its molecular form and its ionic form, H+.
The hydrogen cycle is made up of hydrogen exchanges between biotic (living) and abiotic (non-living) hydrogen-containing compound sources and sinks. The most abundant element in the universe is hydrogen (H). Water (H2O), hydrogen gas (H2), hydrogen sulfide (H2S), and ammonia are examples of common H-containing inorganic molecules on Earth (NH3). Many organic compounds, such as hydrocarbons and organic matter, contain H atoms. Because hydrogen atoms are ubiquitous in inorganic and organic chemical compounds, the hydrogen cycle focuses on molecular hydrogen, H2.
The hydrogen cycle has four main processes:
- Production: Hydrogen is produced through various natural processes, including photosynthesis and the breakdown of organic matter.
- Transport: Hydrogen can be transported in the atmosphere as molecular hydrogen gas or as part of other molecules such as water vapor, methane, and ammonia.
- Transformation: Hydrogen can undergo a variety of chemical reactions, including oxidation and reduction, which can transform it into other forms.
- Deposition: Hydrogen is deposited back into the environment through a variety of processes, including precipitation, chemical reactions, and biological processes.
The hydrogen cycle is important for a variety of reasons. For example, it plays a key role in the Earth’s climate system, as hydrogen is a component of greenhouse gases like water vapor and methane. The hydrogen cycle also has important implications for the search for extraterrestrial life, as hydrogen is a key element for life as we know it.
Hydrogen gas can be produced naturally as a byproduct of microbial metabolisms or as a result of rock-water interactions. Free H2 is then consumed by other microbes, photochemically oxidized in the atmosphere, or lost to space. Hydrogen is also thought to play an important role in pre-biotic chemistry and the early evolution of life on Earth, as well as possibly elsewhere in the Solar System.
Water in the form of precipitation falls back to the Earth’s surface, replenishing the hydrosphere and providing a source of water for plants and other organisms in the biosphere. Hydrogen is also present in organic matter, and as plants and animals die, their remains can become buried in the lithosphere. Over time, this organic matter can be converted into fossil fuels such as coal, oil, and natural gas, which contain hydrogen.
Finally, the hydrogen cycle is completed when these fossil fuels are burned, releasing hydrogen back into the atmosphere in the form of water vapor. This process can also release carbon dioxide and other pollutants, contributing to global climate change and air pollution.