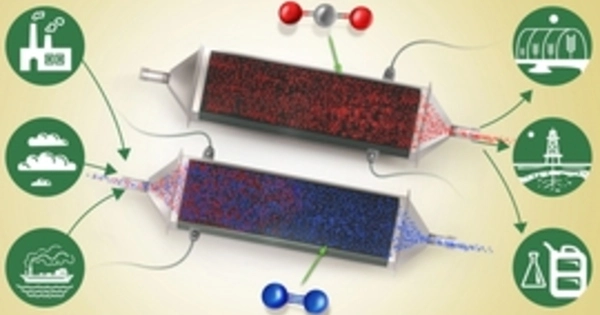
Researchers believe they have discovered the key to a truly efficient and low-cost mechanism for removing carbon dioxide from seawater. The method could be far more efficient than current methods for removing greenhouse gases from the atmosphere.
As carbon dioxide levels in the Earth’s atmosphere continue to rise, scientists around the world have spent years looking for ways to efficiently remove the gas. Meanwhile, the ocean is the world’s number one “sink” for carbon dioxide from the atmosphere, absorbing 30 to 40% of all gas produced by human activity.
Recently, the possibility of removing carbon dioxide directly from ocean water has emerged as another promising option for reducing CO2 emissions, one that could eventually lead to overall net negative emissions. However, as with air capture systems, the concept has yet to see widespread adoption, despite the efforts of a few companies.
A team of MIT researchers believes they have discovered the key to a truly efficient and low-cost removal mechanism. The findings were published this week in the journal Energy and Environmental Science in a paper co-authored by MIT professors T. Alan Hatton and Kripa Varanasi, postdoc Seoni Kim, and graduate students Michael Nitzsche, Simon Rufer, and Jack Lake.
We will not be able to treat the entire planet’s emissions. However, reinjection may be done in some cases in places such as fish farms, which tend to acidify the water, so this could be a way of helping to counteract that effect.
Kripa Varanasi
Existing carbon dioxide removal methods use a voltage across a stack of membranes to acidify a feed stream via water splitting. This converts the bicarbonates in the water to CO2 molecules, which can then be extracted under vacuum. Hatton, the Ralph Landau Professor of Chemical Engineering, points out that the membranes are costly, and chemicals are needed to drive the overall electrode reactions at either end of the stack, adding to the cost and complexity of the processes. “We wanted to avoid using membranes and introducing chemicals into the anode and cathode half cells as much as possible,” he says.
The team devised a reversible process based on membrane-free electrochemical cells. Reactive electrodes are used to release protons into the seawater fed to the cells, causing the dissolved carbon dioxide in the water to be released. The process is cyclic: first, the water is acidified to convert dissolved inorganic bicarbonates to molecular carbon dioxide, which is then collected as a gas under vacuum. The water is then passed through a second set of cells with a reversed voltage to recover the protons and return the acidic water to the sea. Once one set of electrodes has been depleted of protons (during acidification) and the other has been regenerated during alkalization, the roles of the two cells are reversed on a regular basis.
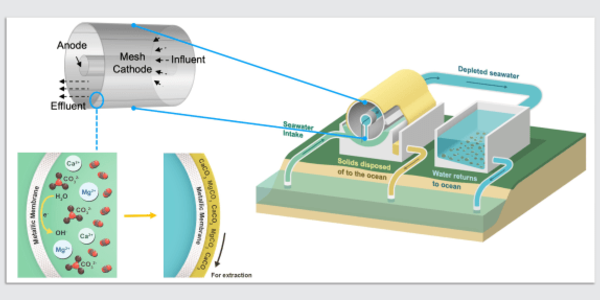
According to Varanasi, a mechanical engineering professor, the removal of carbon dioxide and reinjection of alkaline water could gradually begin to reverse, at least locally, the acidification of the oceans caused by carbon dioxide buildup, which has threatened coral reefs and shellfish. They propose reinjecting alkaline water through dispersed outlets or far offshore to avoid a local spike in alkalinity that could disrupt ecosystems.
“We will not be able to treat the entire planet’s emissions,” Varanasi says. However, reinjection may be done in some cases in places such as fish farms, which tend to acidify the water, so this could be a way of helping to counteract that effect.
Once the carbon dioxide is removed from the water, it still needs to be disposed of, as with other carbon removal processes. For example, it can be buried in deep geologic formations under the sea floor, or it can be chemically converted into a compound like ethanol, which can be used as a transportation fuel, or into other specialty chemicals. “You can certainly consider using the captured CO2 as a feedstock for chemicals or materials production, but you’re not going to be able to use all of it as a feedstock,” says Hatton. “You’ll run out of markets for all the products you produce, so not matter what, a significant amount of the captured CO2 will need to be buried underground.”
At first, the idea would be to combine such systems with existing or planned infrastructure that already processes seawater, such as desalination plants. “This system is scalable, so we could potentially integrate it into existing processes that are already processing or in contact with ocean water,” Varanasi says. There, carbon dioxide removal could be a simple add-on to existing processes that already return vast amounts of water to the sea, and it would not require consumables such as chemical additives or membranes.
“With desalination plants, you’re already pumping all the water, so why not co-locate there?” Varanasi says. “A bunch of capital costs associated with the way you move the water, and the permitting, all that could already be taken care of.”
Ships that process water as they travel could also use the system to help mitigate the significant contribution of ship traffic to overall emissions. There are already international mandates in place to reduce shipping emissions, and “this could help shipping companies offset some of their emissions and turn ships into ocean scrubbers,” says Varanasi.
The system could also be used in places like offshore drilling platforms or aquaculture farms. It could eventually lead to the deployment of free-standing carbon removal plants all over the world.
Hatton believes the process could be more efficient than air-capture systems because the concentration of carbon dioxide in seawater is more than 100 times higher than in air. In direct air-capture systems, the gas must first be captured and concentrated before it can be recovered. “However, because the oceans are large carbon sinks, the capture step has already been done for you,” he says. “There is no capture step; there is only release.” This means that the volumes of material that must be handled are much smaller, potentially simplifying the process and reducing the required footprint.
The investigation is still ongoing, with one goal being to find an alternative to the current step, which requires a vacuum to remove the separated carbon dioxide from the water. Another requirement is to identify operating strategies to prevent mineral precipitation, which can foul the electrodes in the alkalinization cell and reduce overall efficiency in all reported approaches. Hatton observes that while significant progress has been made on these issues, it is still too early to report on them. The team anticipates that the system will be ready for a practical demonstration project in two years.