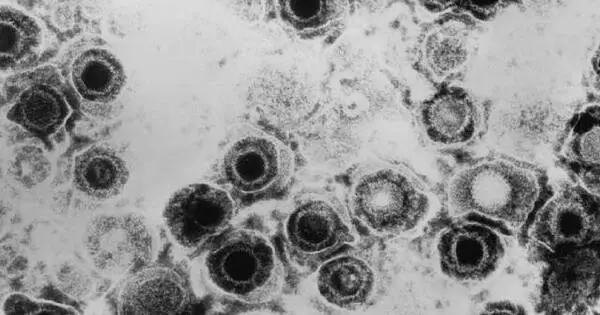
Close contact allows Herpes simplex viruses to spread from person to person. Touching a herpes sore can transmit the herpes simplex virus. Most people, however, contract herpes simplex from a person who is infected but does not have sores. HSV-1 (herpes simplex type 1) is most commonly contracted as an infant or child. This virus is spread through skin-to-skin contact with an adult who has the virus. It is not necessary for an adult to have sores to spread the virus.
HSV1 (herpes simplex virus type 1), which hibernates in the peripheral nervous system and can never be eradicated, is carried by more than half of all adults in the United States. A new study has revealed herpes’ sneaky strategy for infecting the nervous system, paving the way for the development of long-needed vaccines for both HSV1 and its close sibling HSV2.
Herpes type 1 is sealed for life with a kiss. HSV1 (herpes simplex virus type 1), which hibernates in the peripheral nervous system and can never be eradicated, is carried by more than half of all adults in the United States.
A new Northwestern Medicine study has revealed the virus’s sneaky strategy for infecting the nervous system, paving the way for the development of long-needed vaccines for both HSV1 and its close sibling HSV2. Some carriers will never even get a cold sore as a result of HSV1. For others, however, it can result in blindness or life-threatening encephalitis. There is mounting evidence that it contributes to dementia.
By learning how the virus is achieving this incredible feat to get into our nervous system, we can now think about how to take away that ability. If you can stop it from assimilating kinesin, you would have a virus that couldn’t infect the nervous system. And then you have a candidate for a preventive vaccine.
Greg Smith
And HSV2, which is more commonly transmitted through sexual contact, can be passed from mother to newborn as neonatal herpes, causing lesions all over the infant’s body. Most babies recover, but in severe cases, it can cause brain damage or spread to all organs and be fatal.
“We desperately need a vaccine to prevent herpes from invading the nervous system,” said Greg Smith, professor of microbiology and immunology at Northwestern University Feinberg School of Medicine.
The new Northwestern Medicine study from Smith’s lab has uncovered a route to that. The study discovered how herpes kidnaps a protein from epithelial cells and turns it into a defector to help it travel into the peripheral nervous system. They have termed the process “assimilation.” It’s a discovery that may have wide-ranging implications for many viruses, including HIV and SARS-CoV-2, Smith said.
The study will be published in Nature.
Riding the rails
“The virus must inject its genetic code into the nucleus in order to begin producing more herpes viruses,” Smith explained. “It reprograms the cell to turn it into a virus factory. The big question is how it gets to a neuron’s nucleus.”
Herpes, like many viruses, moves along the cell’s train tracks called microtubules by using protein engines called dynein and kinesin. Smith’s team discovered that herpes uses a kinesin engine brought from other cells to ferry itself to the nucleus of the neuron. That kinesin protein acts as a defector for the virus’s benefit.
“By learning how the virus is achieving this incredible feat to get into our nervous system, we can now think about how to take away that ability,” Smith said. “If you can stop it from assimilating kinesin, you would have a virus that couldn’t infect the nervous system. And then you have a candidate for a preventive vaccine.”
Herpes takes a ‘cross-country’ trip
Consider the cell to be a rail yard. All tracks lead to a central hub known as the centrosome. Train engines are classified into two types: dynein and kinesin proteins. One leads to the hub (say, downtown), while the other leads away from it to the suburbs.
When a more common virus, such as influenza, infects mucosal epithelial cells (cells that line your nose and mouth), it latches onto both engines and moves back and forth on the microtubule tracts until it eventually ends up in the nucleus by chance. Overall, the commute from the suburbs to the nucleus via the centrosome is short.
However, traveling down nerves is the equivalent of a cross-country trip. Herpes uses the dynein engine for this trip, but it also ensures that the kinesin engines do not take it back the way it came.
“There’s a long way to go,” Smith said. “It probably takes eight hours to travel from the end of the neuron to the hub.” However, the dynein engine can only take it as far as the hub. Herpes must enter the nucleus. That’s when it reaches into its ‘pocket’ and pulls out a kinesin engine that it kidnapped from mucosal epithelial cells and convinced to join its team. And, in a betrayal, that assimilated kinesin transports it directly to the nucleus.
“This is the first time a virus has been discovered repurposing a cellular protein and using it to drive subsequent rounds of infection,” said Caitlin Pegg, a graduate student in Smith’s lab. “We’re excited to learn more about the molecular mechanisms that have evolved to make these viruses arguably the most successful pathogens known to science,” Smith said.