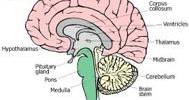
Decentralized clinical trials were likely on the horizon before the epidemic pushed distant employment, school, and research to the forefront. They have arrived in earnest now. Alto Neuroscience, a precision psychiatry business, and Cerebral, an online mental health service, unveiled a decentralized Phase 2 clinical study for Alto Neuroscience’s depression medication candidate, ALTO-300, this week. The majority of the research will take place in the patients’ homes.
The initiative will specifically recruit 200 participants from the cerebral platform who are currently suffering from depression and have found no relief from conventional therapies. Apart from offering the new medicine, Alto Neuroscience plans to verify its drug development strategy, which involves utilizing patient biomarkers to forecast which treatments patients will (or will not) respond to.
“The concept of performing deep phenotyping on a patient population and figuring out which subgroups of patients truly benefit from the therapy before spending a billion dollars on clinical trials made all the sense in the world,” David Mou, Cerebral’s chief medical officer, told. “It was a match made in heaven in a way.” We had everything they needed, and I’m confident that their concept will be the most practical.”
A “decentralized clinical trial” is defined in several ways, but in essence, it implies that treatment is delivered to the patient in some way, whether online or by mobile doctors. In addition, rather than visiting a research facility on a regular basis, data is generally collected where patients are. Bringing clinical trials to patients has the potential to overcome some of the key issues that now plague clinical trials by making the procedure more user-friendly. For example, over 70% of clinical trial participants reside more than two hours from a study facility.
Studies also frequently stopped due to enrollment issues, with an estimated 80% of clinical trials failing to recruit participants on schedule. Finally, experts have proposed that bringing trials to patients might assist increase drug research’s diversity and accessibility.
Although this is far from the first decentralized clinical study, it does occur at a critical juncture in the field. Only 38% of pharma and contract research companies (CROs) informed McKinsey that decentralized clinical trials will be a significant part of their portfolios before the pandemic. When McKinsey questioned those same firms again in 2020, they all predicted that decentralized trials would play a significant role.