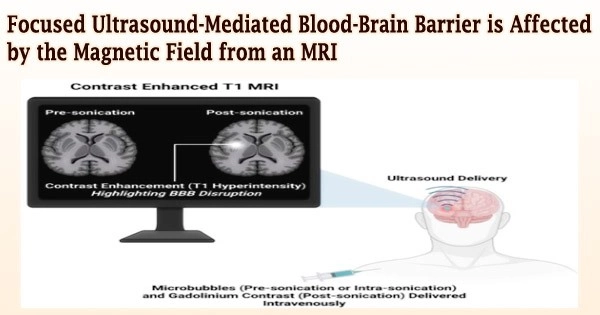
The blood-brain barrier (BBB) can be opened using MRI-guided focused ultrasound in conjunction with microbubbles, allowing therapeutic medications to enter the sick brain area. It is a promising method that has been found to be safe in patients with a variety of brain disorders, including glioblastoma, Parkinson’s disease, Alzheimer’s disease, and others.
Although preclinical research and clinical studies have frequently employed MRI for treatment guidance and assessment, until today, researchers were unaware of how the static magnetic field produced by the MRI scanner affected the size of the BBB opening and the effectiveness of medication transport.
Hong Chen and her team at Washington University in St. Louis have discovered for the first time in new research published in Radiology that the magnetic field of the MRI scanner reduced the BBB opening volume in a mouse model by 3.3-fold to 11.7-fold depending on the strength of the magnetic field.
The study was conducted on 30 mice divided into four groups by Chen, an associate professor of radiation oncology and biomedical engineering in the School of Medicine and McKelvey School of Engineering.
Following the injection of the microbubbles, three groups of mice received focused-ultrasound sonication at magnetic field strengths of 1.5 T, 3 T, and 4.7 T, while one group was not exposed to the magnetic field at all.
In comparison to individuals who had received the dose outside of the magnetic field, they discovered that the activity of the microbubble cavitation, or the expansion, contraction, and collapse of the microbubbles, dropped by 2.1 decibels at 1.5 T, 2.9 decibels at 3 T, and 3 decibels at 4.7 T. Additionally, at 1.5 T, 3 T, and 4.7 T, the magnetic field reduced the BBB opening volume by 3.3, 4.4, and 11.7 times, respectively.
The dampening effect of the magnetic field on the microbubble is likely caused by the loss of bubble kinetic energy due to the Lorentz force acting on the moving charged lipid molecules on the microbubble shell and dipolar water molecules surrounding the microbubbles.
Yaoheng (Mack) Yang
No tissue damage from the operation was visible in any of the animals. The scientists employed the model drug Evans blue to evaluate if the static magnetic field has an impact on the effectiveness of trans-BBB drug transport after focused-ultrasound sonication.
According to the photos, mice treated in one of the three intensities of magnetic fields had lower levels of Evans blue fluorescence than mice treated outside the magnetic field.
At 1.5 T, 3.0 T, and 4.7 T, the Evans blue trans-BBB delivery was reduced by 1.4, 1.6, and 1.9 times, respectively, in comparison to individuals who were not exposed to the magnetic field.
“The dampening effect of the magnetic field on the microbubble is likely caused by the loss of bubble kinetic energy due to the Lorentz force acting on the moving charged lipid molecules on the microbubble shell and dipolar water molecules surrounding the microbubbles,” said Yaoheng (Mack) Yang, a doctoral student in Chen’s lab and the lead author of the study.
“Findings from this study suggest that the impact of the magnetic field needs to be considered in the clinical applications of focused ultrasound in brain drug delivery,” Chen said.
Cavitation is the essential physical process for a number of additional therapeutic approaches, including sonothrombolysis, a treatment for acute ischemic stroke, and histotripsy, which uses cavitation to mechanically destroy areas of tissue.
When MRI-guided focused-ultrasound systems are utilized, it is anticipated that the dampening effect on cavitation caused by the magnetic field may impair the treatment outcomes of other cavitation-mediated procedures.