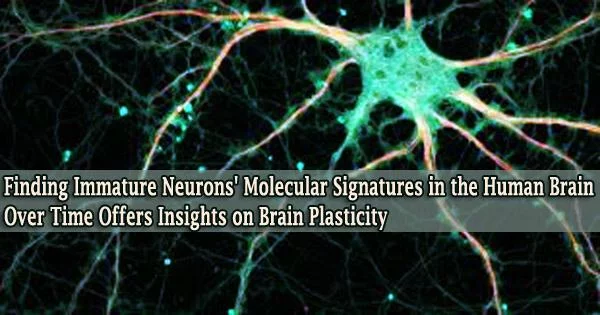
In a crucial memory area of the brain called the hippocampus, researchers from the Perelman School of Medicine at the University of Pennsylvania led a team that employed cutting-edge methods to demonstrate that immature, malleable neurons are present in considerable numbers throughout the human lifespan.
The research, which was just published in Nature, aims to put an end to a long-running debate over the reality of “adult neurogenesis,” or the generation of new, immature neurons in the developed human brain. The discovery opens up new opportunities for investigating adult neurogenesis’ functions in memory, emotion, behavior, and brain illnesses.
“Many mammals generate new neurons in their brains throughout their lifespans which play a critical role in the brain’s plasticity, or ability to change and adapt over time. This ability to repair itself is especially important when the brain is damaged, which is what happens during a stroke or brain injury,” said senior author Hongjun Song, PhD, a Perelman Professor of Neuroscience at Penn. “This plasticity is also important for understanding diseases like Alzheimer’s, which affect a patient’s memory, among other functions.”
There has been discussion on adult neurogenesis in humans for many years. Neuroscientists held the belief for almost a century that since no new neurons were created in the mammalian brain when it reached adulthood, the ones that were already there had to continue functioning.
Eventually, research started to show that adult brains from mice, humans, and other mammals contain newly formed, immature neurons, particularly in the hippocampus and olfactory (smell) region.
Since the hippocampus plays important functions in learning, memory, and mood regulation and is diminished in the brains of persons with Alzheimer’s disease, immature neurons in this brain region have drawn a lot of attention and continue to do so.
Other research conducted over the past few years, however, have not discovered any proof of considerable adult neurogenesis in the human hippocampus. Because they lacked a simple, sensitive, and precise method for identifying recently formed, immature neurons in samples of adult human brain tissue, neuroscientists have struggled to resolve the controversy.
Many mammals generate new neurons in their brains throughout their lifespans which play a critical role in the brain’s plasticity, or ability to change and adapt over time. This ability to repair itself is especially important when the brain is damaged, which is what happens during a stroke or brain injury. This plasticity is also important for understanding diseases like Alzheimer’s, which affect a patient’s memory, among other functions.
Professor Hongjun Song
Two potent and relatively recent tools helped Song, Ming, and their team overcome this obstacle. Single-nucleus RNA sequencing is the first method, which captures nearly all of the gene activity in a single cell.
The second is machine learning, a form of artificial intelligence that was utilized in this study to comb through enormous gene-activity datasets for mice and humans to discover the minute variations between mature and immature hippocampal neurons.
These techniques allowed the researchers to establish the existence of immature hippocampus neurons, primarily of a kind known as granule cells, in a variety of human brain samples, ranging in age from infancy to 92.
Even in old brains, the immature granule cell population was typically at least a few percent of the total population. Other areas of adult human brains were not found to contain appreciable numbers of immature neurons by the researchers.
The investigation revealed a broad pattern of immature granule cell-specific gene activity and demonstrated how this pattern changes with healthy aging, varies between humans and mice, and is disrupted in Alzheimer’s disease.
The researchers discovered that, in Alzheimer’s brains compared to age-matched non-brains, Alzheimer’s the proportion of immature granule cells among all granule cells was significantly reduced by more than half.
The researchers examined the expression of known risk genes for brain disorders including Alzheimer’s and autism spectrum disorders in immature granule cells over the lifespan as further indication of the power of this type of analysis for determining the origins of disease.
They discovered that a number of these risk genes started to express themselves in developing granule cells at ages when the associated illness is likely to first appear.
The study involved the characterization of the neural stem cells that give rise to new granule cells in the hippocampus, known as hippocampal progenitor cells. These studies showed that although the progenitors are very infrequent, they are a reliable source of fresh granule cells, which develop slowly over the course of a year or more.
“In the future, we hope this type of research can help to further understand the causes of brain diseases like psychiatric disorders and Alzheimer’s, which can inform the possibility of treating these diseases,” said senior author Guo-li Ming, MD, PhD, a Perelman Professor of Neuroscience.
As well as contributions from tissue banks across the nation, the research was made feasible through tight interactions with the neurosurgery teams at Penn and CHOP.
The research was supported by the National Institutes of Health (R35NS097370, R35NS116843), the Lieber Institute for Brain Development, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation.