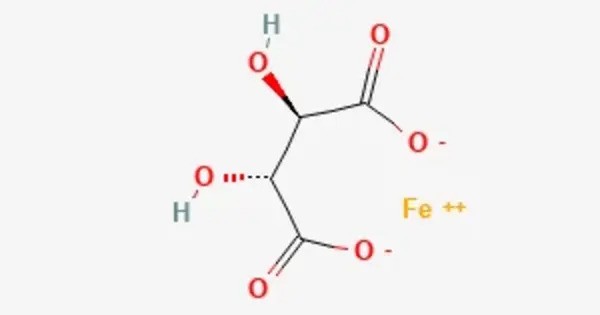
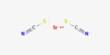
Ferrous tartrate is a chemical compound and the iron(II) salt of tartaric acid. It is the iron(II) salt of tartaric acid. Historically used as a medicinal tonic in the 19th and early 20th centuries, it was primarily prescribed to treat anemia and as a stomachic, with typical doses of 1-2 fluid drams for anemia or 2 tablespoons as a tonic. It was often prepared by digesting 2 ounces of tartarated iron in a pint of sherry for 30 days, a process noted for its difficulty. The compound, with a molar mass of approximately 203.915 g/mol, can form hydrated versions and is sensitive to pH, affecting its stability.
Ferrous tartrate releases bioavailable iron ions, aiding hemoglobin synthesis, and may exhibit antioxidant properties. Beyond medicine, it finds applications in analytical chemistry for colorimetric tannin assays, in pharmaceuticals as an iron supplement, and in material science for iron-based nanomaterials. It can form complexes with organic compounds, influencing their reactivity, and is synthesized by reacting iron(II) salts with tartaric acid. Its historical use as a “steel medicine” highlights its role in early iron therapy.
Properties
- Chemical formula: C4H4FeO6
- Molar mass: 203.92 g/mol
- Appearance: Reddish powder
- Solubility: Soluble in water, which facilitates its use in biochemical and medical applications.
- Biological Activity: It releases iron ions in physiological conditions, contributing to hemoglobin synthesis, and may exhibit antioxidant properties.
Structure
Ferrous tartrate is the iron(II) salt of tartaric acid, classified as an organic transition metal salt. It consists of iron(II) ions bonded to tartrate ions, derived from tartaric acid, a dicarboxylic acid with hydroxyl groups on the C2 and C3 carbon atoms.
Historical uses
Ferrous tartrate has been used as a steel medicine. It was generally prescribed during the 19th and early 20th centuries. It is usually prepared by digesting for 30 days, 2 ounces (880 grains) tartarated iron in a pint of sherry. It can be difficult to prepare.
Historically, it was used as a stomachic and tonic, at a dose of 2 tbsp. It was also used to treat anemia, dose 1 to 2 fl. dr.
Synthesis
Ferrous tartrate is synthesized by reacting iron(II) salts (e.g., iron(II) sulfate or chloride) with tartaric acid under controlled pH conditions. Another method involves using sodium metasilicate to facilitate the formation of ferrous tartrate nanoparticles. Historically, it was prepared by digesting tartarated iron (880 grains) in a pint of sherry for 30 days, a process noted for its difficulty.
Modern Applications
Scientific Research: Ferrous tartrate is used in biochemical studies, particularly for its role in redox reactions and complexation with organic compounds, influencing stability and reactivity. It serves as a catalyst in certain chemical syntheses.
Nanoparticle Research: Studies explore its nanoparticle forms for potential use in iron supplementation, leveraging its bioavailability.
Occurrences
Natural Occurrence: Ferrous tartrate does not occur naturally in significant quantities but is synthesized for specific applications. It is not typically found in geological or environmental contexts like some other iron compounds (e.g., magnetite or ferrihydrite).
Synthetic Contexts: It is primarily encountered in laboratory settings, pharmaceutical preparations, or historical medicinal formulations. Its presence in modern contexts is limited to research and niche chemical applications.
Safety and Toxicity
Specific safety data for ferrous tartrate is limited in the provided sources, but as an iron supplement, it is generally considered safe in controlled doses. Excessive iron intake can lead to toxicity, a concern with all iron-based compounds.