Metal is a durable material that has been used by humans for six thousands of years since it takes a lot of energy to harm it. The disadvantage of this ability is that it takes a lot of energy to fix that damage. Typically, welding torches with a maximum temperature of 6,300 °F are used to melt the metal during the repair procedure.
Penn Engineers have now created a method for repairing metal at ambient temperature for the first time. They refer to their method as “healing” because it works in a manner akin to how bones recover by obtaining raw materials and energy from an outside source.
The study was conducted by James Pikul, assistant professor in the Department of Mechanical Engineering and Applied Mechanics and Zakaria Hsain, a graduate student in his lab.
It was published in the journal Advanced Functional Materials.
In addition to the energy expenses involved in the existing method of repairing metal by melting it into a more malleable shape, some metal components do not even allow for such a restoration plan.
For instance, metallic foams, which are metals constructed with internal air pockets, lose their complex internal structure when they are melted. The material’s weight is decreased while overall strength is maintained by this configuration of struts and gaps.
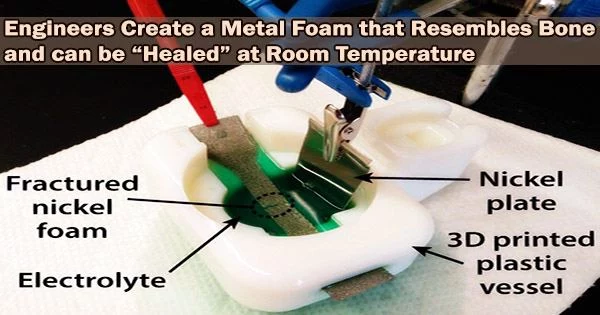
The electrolyte fluid that allows the healing can be integrated into the metal foams so that it resembles blood in our body. Once the foam is fractured, the electrolyte will surround the fractured area and heal the metal after the application of an external voltage, which can be from a battery.
James Pikul
While exploring ways to repair such porous metals, Pikul and Hsain looked at existing “self-healing” materials, which are generally made from relatively soft polymers and plastics.
“The way people do self-healing today is they impregnate these polymers with different chemicals that, when that polymer is ruptured, are released and mix like an epoxy, gluing the material back together,” Pikul says. “That approach works for polymers because polymers can flow and are relatively easy to reshape at room temperature, but that means they have limited strength as a result.”
Pikul and Hsain began by figuring out a technique for metal foams to “sense” where they had been injured in order to repair them because they often have greater structural qualities than polymers. The researchers came to the conclusion that they may use the breaking of a polymer layer as a form of chemical signal rather than encapsulating extra chemicals employed in healing.
Pikul and Hsain used chemical vapor deposition to evenly coat each strut of the nickel foam with a layer of Parylene D, a chemically inert and stretchy polymer. The damage tolerance of this substance is slightly lower than that of nickel, thus as the sample is damaged, it breaks first, exposing the metal beneath. The new nickel struts could then be constructed by the researchers using electroplating solely on the exposed nickel where it was required.
Electroplating is a low-energy, room-temperature process that is frequently used to add a layer of gold or chrome to jewelry or automobile parts. In the earlier illustration, a steel tire rim is submerged in a solution of chromium ions as an electrolyte. Ions nearby the steel react when a voltage is provided, covering it uniformly with chromium metal.
“Unlike polymers, metals don’t flow at room temperature,” Pikul says. “The nice thing with electrochemistry is that metal ions can easily move through the liquid electrolyte. We then use electrochemistry to convert the ions to solid metal. The polymer acts like a lithography mask and only allows the ions to turn into metal where the metal foam was broken.”
Pikul and Hsain healed three types of damage in their experiments on centimeter-scale samples of their polymer-coated nickel foam: samples with cracks, samples that had been pulled apart until they were connected by just a few struts, and samples that had been cleaved in two.
It took almost four hours to repair the damage, and since electroplating operates on all of the exposed nickel at once, the amount of time required is independent of the size of the sample.
Pikul believes that this room-temperature method is in keeping with how self-healing happens in the body, even though it is not fully “self-healing” because it needs an outside power source and raw materials.
“I think most people would say bone is a self-healing material,” Pikul says, “and I think, in practice, our material is very similar to bone. Bone is not fully self-contained either; it needs an energy source and nutrients to heal, both of which come from eating food. In our system, these function similarly to the voltage and electroplating bath.”
Similar to bone, where additional nickel grows at the wound site, the mended parts are actually stronger than they were before they were harmed. When applying this approach repeatedly, the new nickel, however, decreases the effectiveness of healing. Should another component of the sample need to be repaired, nickel would keep accumulating there because the healed areas no longer have a polymer coating.
Pikul hopes that further research on this technique will increase the similarities to biological healing.
“The electrolyte fluid that allows the healing can be integrated into the metal foams so that it resembles blood in our body,” Pikul says. “Once the foam is fractured, the electrolyte will surround the fractured area and heal the metal after the application of an external voltage, which can be from a battery.”
Without having to remove and immerse the broken part, the foam might be repaired, which is especially helpful if the item in question is a car door, robotic arm, or space station component.