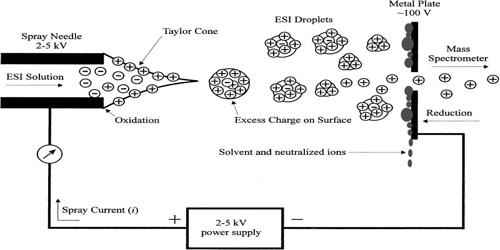
Electrospray is an electrohydrodynamic technique similar to electrospinning. Electrospray ionization (ESI) is a technique used in mass spectrometry to produce ions using an electrospray in which a high voltage is applied to a liquid to create an aerosol. ESI requires sample introduction in liquid form and thus is convenient for the analysis of oligonucleotides. It is especially useful in producing ions from macromolecules because it overcomes the propensity of these molecules to fragment when ionized. While ESI is widely used, it is subject to matrix effects, particularly ion suppression, which must be taken into consideration during method development. ESI is different from other ionization processes since it may produce multiple-charged ions, effectively extending the mass range of the analyzer to accommodate the kDa-MDa orders of the magnitude observed in proteins and their associated polypeptide fragments. ESI generates gaseous ions from polar compounds in liquid solvents passing through a capillary tip on which a strong electric field is applied
ESI generates intact gas-phase ions from analytes in a solution for mass spectrometric investigations. Mass spectrometry using ESI is called electrospray ionization mass spectrometry (ESI-MS) or, less commonly, electrospray mass spectrometry (ES-MS). ESI is perhaps the most commonly used ionization technique in clinical MS. It is a sensitive ionization technique for analytes that exist as ions in the LC eluent. ESI is a so-called ‘soft ionization’ technique since there is very little fragmentation. The implementation of the ESI source on a mass spectrometer is achieved by using an ESI–MS interface. This can be advantageous in the sense that the molecular ion (or more accurately a pseudo molecular ion) is always observed, however very little structural information can be gained from the simple mass spectrum obtained. This disadvantage can be overcome by coupling ESI with tandem mass spectrometry (ESI-MS/MS). Another important advantage of ESI is that solution-phase information can be retained into the gas-phase.
Advantages
- Its ability to handle samples that have large masses.
- This ionization method is one of the softest ionization methods available.
- It has the ability to analyze biological samples that are defined by non-covalent interactions.
- The sensitivity for this instrument is impressive and therefore can be useful in accurate quantitative and qualitative measurements.
Disadvantages
- A major disadvantage is that this technique cannot analyze mixtures very well, and when forced to do so, the results are unreliable.
- The apparatus is also very difficult to clean and has a tendency to become overly contaminated with residues from previous experiments.
- Finally, the multiple charges that are attached to the molecular ions can make for confusing spectral data.
ESI source is another important soft ionization method used for biomolecule analysis. ESI can proceed via different mechanisms. Low molecular weight analytes follow the ion evaporation model (IEM), whereas the charged residue model (CRM) applies to large globular species. The electrospray ionization technique was first reported by Masamichi Yamashita and John Fenn in 1984. The development of electrospray ionization for the analysis of biological macromolecules was rewarded with the attribution of the Nobel Prize in Chemistry to John Bennett Fenn in 2002. One of the original instruments used by Dr. Fenn is on display at the Science History Institute in Philadelphia, Pennsylvania.