A failed pesticide screening can just as easily kill your entire crop as the pests that were attacking it! There are alternatives to using chemicals to control pests. Beneficial insects and predatory mites can be of assistance. The Russian Wheat Aphid, Diamond Back Moth, and Potato Leaf Hopper are among the pest insects targeted, and the current candidate fungi are Beauveria bassiana, Paecilomyces fumosoroseus, and Zoophthora radicans.
Compounds that influence insect behavior by modulating their ability to smell could be developed by targeting their chemoreceptors, according to an emerging concept in the ongoing battle against insect pests.
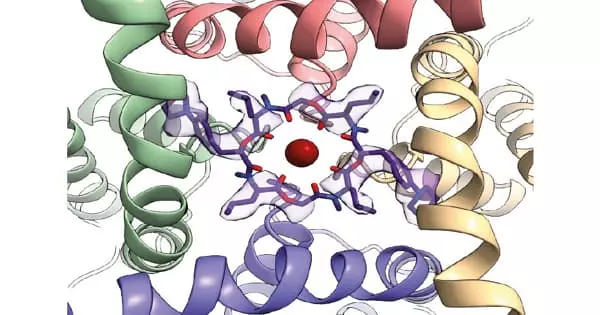
Ion channels in the nervous system are among the most important insecticide targets. Understanding the structure of the channels is critical for identifying novel species-specific agrochemical binding sites. The structure and function of a potassium ion channel in fruit flies have been discovered by scientists. Their new discoveries shed light on the differences between human and insect channels, explain how known compounds affect the channel, and suggest new drug target sites. The findings could aid pesticide manufacturers in developing new drugs that specifically kill pest insects and parasites while not harming other animals such as bees and mammals.
Ideally, you want insecticides to be very specific to the pest insect, avoiding drugs that are toxic to humans or other animals like birds, rodents, and beneficial insects like bees. The difference between human and insect channels is really small, but we found protein regions that are specific to insects.
Stefan Raunser
Slowpoke potassium channels are huge and complex proteins that sit inside the cellular membrane and selectively and rapidly transport vital potassium ions through it in Drosophila, the common fruit fly. They are found in all animals and are in charge of a variety of tasks, most notably in the brain and muscle cells.
The essential roles of the potassium channels highlight the importance of targeting Slowpoke with newly developed insecticides to aid in overcoming the global problem of decreasing efficiency due to pesticide resistance. However, there is always the risk of not aiming correctly: “Ideally, you want insecticides to be very specific to the pest insect, avoiding drugs that are toxic to humans or other animals like birds, rodents, and beneficial insects like bees,” says Stefan Raunser, Director of the Max Planck Institute of Molecular Physiology in Dortmund and lead author of the study.

Scientists require high-resolution ion channel structures in order to design drugs that are specific for pest insects. Raunser and colleagues used cryo-electron microscopy (cryo-EM) to obtain the protein’s open and closed structures and compared them to previously known human protein structures. “The difference between human and insect channels is really small,” says Raunser, “but we found protein regions that are specific to insects.”
Each year, approximately 545 million kg of pesticides are applied to crops in the United States, with 20% being insecticides, 68% being herbicides, and 12% being fungicides for pest control. Despite this heavy and costly pesticide application, pests destroy an estimated 37% of all US crops, while treated crop losses worldwide are around 50%.
Detailed map of the potassium channel for drug discovery
The channel’s RCK2 pocket contains amino acids that differ between Drosophila and humans. It’s at the bottom of the channel, near the gating ring. The gating ring sits inside the cell, picks up calcium ions when they are abundant, and initiates a series of rearrangements that allow potassium ions to pass through the central cavity. The shape of the RCK2 pocket changes as it transitions between closed and open states.
As a result, it is a potentially ideal target for small molecules that can block the channel in either state. Other, less insect-specific drug target sites were also identified by the researchers. Among them, the S6 pocket is closed and could be used to lock the channel. “We’re giving pharmaceutical scientists a detailed map of the potassium channel so they can make better, more selective insecticides,” Raunser says.
The cryo-EM structures of the channel were also solved by the researchers using two known compounds, verruculogen and emodepside. Verruculogen, a fungal neurotoxin, is a small molecule that fits perfectly in the S6 pocket, close to the central cavity. Verruculogen keeps the channel narrow, preventing it from opening. Another compound that binds close to the S6 pocket is emodepside, a drug used to treat gastrointestinal worms in cats and dogs. However, it behaves differently, acting as an additional passing filter, making it difficult for potassium to pass through the channel optimally. “It’s critical to understand how these ligands manipulate the channel,” Raunser says.