One in five persons have anxiety disorders, which are common mental health concerns. They exhibit varied degrees of trepidation, dread, and panic and are frequently accompanied with catastrophic ideas about fictitious or real threats.
Researchers have found that anxiety affects a wide range of animal species as well, proving that it is not just a human condition. These behaviors can aid animals in avoiding injury and hazardous conditions, thus aiding in their survival. It is now understood that these behaviors serve an evolutionary purpose.
However, in humans, when these behavioral patterns go out of control, they may be extremely disruptive and have a considerable negative influence on daily lives and experiences. Thus, it could be very beneficial to learn more about anxiety and the neurological mechanisms that underlie it.
Most importantly, it might be useful to develop better treatment plans that could enhance the lives of the numerous people with anxiety disorders.
Researchers at Zhejiang University and other universities in China have recently carried out a study on rats aimed at better understanding the serotonergic pathways associated with distinct anxious behaviors.
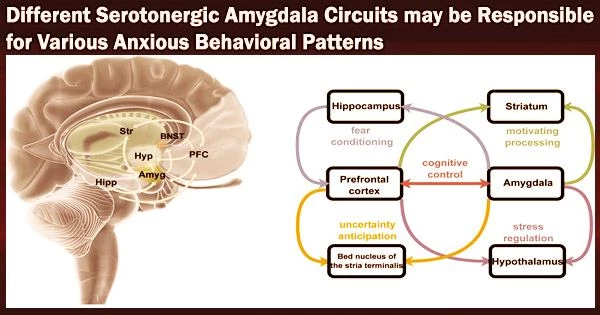
Their findings, published in Nature Neuroscience, suggest that different serotonergic pathways leading to the amygdala, the brain region known to be associated with fear and emotional behavior, could support different behavioral features of anxiety.
In dorsal raphe nucleus (DRN), 5-HT∩vGluT3 neurons projecting to BA parvalbumin (DRN5-HT∩vGluT3-BAPV) and pyramidal (DRN5-HT∩vGluT3-BAPyr) neurons have distinct intrinsic properties and gene expression and respond to anxiogenic and social stimuli, respectively.
Xiao-Dan Yu, Yu Zhu
Mice frequently engage in two crucial behaviors that resemble anxiety while under stress: avoiding contact with other mice and hiding in the dark or avoiding light. In their experiments, the scientists looked at the behaviors that mice exhibited in response to altered serotonergic pathways.
“We demonstrate that in mice, social and anxiogenic stimuli, respectively, increase and decrease serotonin (5-HT) levels in basal amygdala (BA),” Xiao-Dan Yu, Yu Zhu and their colleagues write in their paper. “In dorsal raphe nucleus (DRN), 5-HT∩vGluT3 neurons projecting to BA parvalbumin (DRN5-HT∩vGluT3-BAPV) and pyramidal (DRN5-HT∩vGluT3-BAPyr) neurons have distinct intrinsic properties and gene expression and respond to anxiogenic and social stimuli, respectively.”
“Activation of DRN5-HT∩vGluT3→BAPV inhibits 5-HT release via GABAB receptors on serotonergic terminals in BA, inducing social avoidance and avoidance of bright spaces. Activation of DRN5-HT∩vGluT3→BA neurons inhibits two subsets of BAPyr neurons via 5-HT1A receptors (HTR1A) and 5-HT1B receptors (HTR1B). Pharmacological inhibition of HTR1A and HTR1B in BA induces avoidance of bright spaces and social avoidance, respectively.”
In essence, the researchers discovered that both male and female mice showed somewhat different anxiety-like behavioral patterns when they engaged various serotonergic pathways. Their findings underline the significance of particular serotonergic routes to the amygdala in the control of various anxiety-related behaviors.
More studies on this subject may examine potential gender differences in the expression of these serotonergic pathways in the future, as previous research indicates that female anxiety may be influenced by ovarian cycle hormones.
Additionally, the current work by Yu, Zhu, and their associates may open the door for additional investigation into the serotonergic pathways they looked at, which could support or clarify these findings.