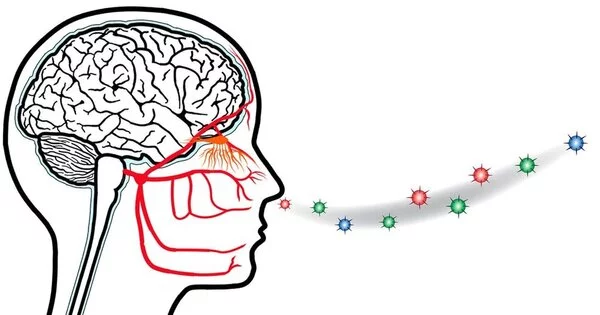
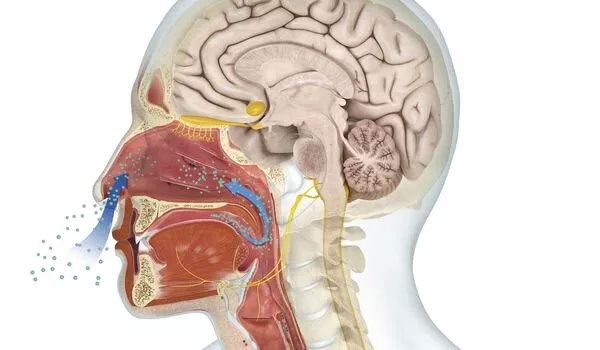
The olfactory bulb, a structure in the front of the brain that sends information to other areas of the body’s central command for further processing, handles smells. Odors travel directly to the limbic system, including the amygdala and hippocampus, which are associated with emotion and memory.
Tufted cells, which are neurons in the brain’s olfactory bulb, have been difficult to study since their discovery more than a century ago. The close proximity of tufted cells to other neurons known as mitral cells limited the ability to dissect the activity of each individual neuron. Cold Spring Harbor Laboratory (CSHL) neuroscientists were able to compare the activity of neurons by using fluorescent genetic markers and new optical imaging technologies.
You can’t track an odor if you can’t tell the difference between high [intensity] and low. You can’t tell if you’re getting closer to the source of the odor if you can’t tell the difference.
Florin Albeanu
CSHL Associate Professor Florin Albeanu and Assistant Professor Arkarup Banerjee discovered tufted cells are better at recognizing smells than mitral cells. They’ve found tufted cells are essential to one of two parallel neural circuit loops that help the brain process different odor features. The findings help explain how the brain takes in sensory information that influences behavior and emotions.
The researchers subjected mice to a variety of odors, ranging from fresh mint to sweet bananas, at varying concentrations. They tracked the neural activity of the two cell types at the same time and discovered that tufted cells outperformed mitral cells. They were faster and more accurate at detecting smells. They also captured a broader spectrum of concentrations. While this revealed a new function for tufted cells, it also raised a new unanswered question. “If tufted cells are better at recognizing odors, what is the function of mitral cells?” Albeanu asked.
Albeanu and Banerjee think mitral cells enhance important smells. They are part of a neural feedback loop that may help an animal prioritize, for example, the smell of food or a predator. In contrast, the tufted cells are part of a second feedback loop that helps process smell intensity and identity. This can guide animals locating odors in the environment. Banerjee explains:
“You can’t track an odor if you can’t tell the difference between high [intensity] and low [intensity]. You can’t tell if you’re getting closer to the source of the odor if you can’t tell the difference.”
The two neural circuit loops provide new insights into how the brain processes sensory data. In the future, the CSHL team’s new genetic and optical imaging tools, which include postdoc Honggoo Chae and graduate student Marie Dussauze, will be able to identify more undervalued neurons involved in sensory processing.
Smell is the most powerful human sense, and, contrary to popular belief, it may be just as powerful as the snout sniffers in dogs and rodents (to certain degrees). Our olfactory receptor neurons, which are located behind your sinuses and are the only neurons in your body that are exposed to air, come into physical contact with the molecules that make up an odor. The information is then immediately transmitted to the brain. Our sense of smell is so powerful that it influences how we perceive taste more than any other sense, even influencing our behavior, emotions, perceptions, and memories.