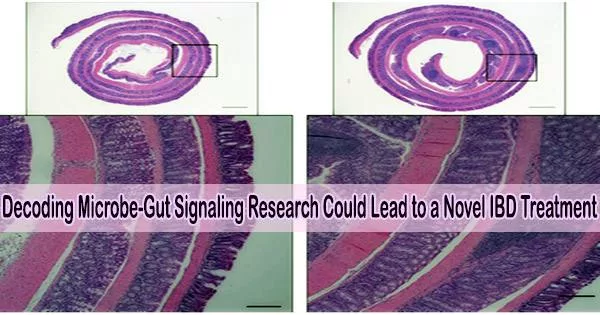
Inflammatory bowel illnesses like Crohn’s disease and ulcerative colitis may soon be treated with a two-drug combination, according to recent discoveries about how our systems interact with the bacteria that live in our guts.
Crohn’s disease is a chronic inflammatory bowel disease (IBD) that primarily affects the gastrointestinal tract. It is characterized by inflammation, which can occur anywhere from the mouth to the anus but most commonly affects the small intestine and the colon.
The study, conducted by professionals at Cincinnati Children’s, was published online on March 28, 2023, in the Journal of Experimental Medicine. Co-first authors were Garrett Overcast, PhD, and Hannah Meibers, BS. Corresponding author was Chandrashekhar Pasare, DVM, PhD, Division of Immunobiology and co-director, Center for Inflammation and Tolerance.
In order to understand how immune cells in the lining of the intestine recognize and react to bacteria as well as transmit vital signals to gut epithelial cells, the research team carried out a number of tests. The immune system and the gut’s friendly bacteria can coexist when the signaling networks between immune cells and epithelial cells are in good working order.
Acting in unhealthy concert
When genetic changes or other factors, such as damage to the intestinal epithelium, muddle microbe-to-cell signals, the immune system may either fail to respond or overreact, which can result in inflammatory bowel disease (IBD).
This study shows that immune cells in the intestines are capable of detecting microorganisms. These immune cells deliver signals to induce a protein called IL-1. This increases levels of another protein called IL-22, which in turn, begins acting in concert with IL-1 to activate the IL-1 receptor (IL-1R) expressed on intestinal epithelial cells.
In addition to activating other genes that draw inflammatory cells to the tissue, activation of the IL-1R also causes the production of ROS. This chain reaction drives an excessive inflammatory response that can damage the intestine, the researchers say.
“The pathogenic role that IL-22 appears to play in inflammatory responses due to its synergy with IL-1R signaling had not been made clear previously,” Pasare says. “We believe this may help explain why past treatments for IBD that focused only on inhibiting IL-1? activity had mixed results. We believe that a combined blockade of both IL-22 and IL-1R could serve as a more promising treatment for IBD.”
What’s next?
Clinical trials have looked at several monoclonal antibodies that can block IL-22 or IL-1R for a variety of auto-immune diseases. The study team is interested in determining whether it is safer to employ currently available medications in combination therapy or whether it would be more beneficial to develop brand-new drugs that specifically target the two pathways.
Cincinnati Children’s co-authors for this publication also included Emily Eshleman, PhD, Irene Saha, PhD, Lisa Waggoner, MS, Krupaben Patel, BS, David Haslam, MD, Theresa Alenghat, VMD, PhD, and Kelli VanDussen, PhD; and Viral Jain, MD, University of Alabama at Birmingham.