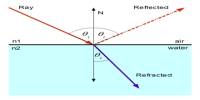
Basic objective of this lecture is to present on Dalton’s Law of Partial Pressures. Dalton found that the total pressure of mixed gases is equal to the sum of their individual pressures (provided the gases do not react). The pressure exerted by an individual gas in a mixture is known as its partial pressure. Example; A balloon is filled with pure oxygen. What is the pressure of the oxygen in the balloon? Ans – Atmospheric pressure. If it wasn’t, then the balloon would expand or shrink.
More Posts
Latest Post
-

Oxprenoate Potassium
-

Crude Oil could be Fractionated with a Revolutionary Method that uses significantly less Energy
-

Electric Aircraft may be made possible via a New Fuel Cell
-

Monopotassium Phosphite
-

Researchers Recently made significant Progress on the Quantum Internet
-

Findings could Improve the Performance of Solid-state Batteries