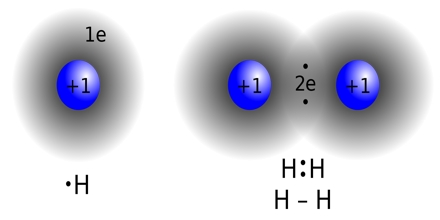
Earlier concepts in covalent bonding arose from these kinds of image of molecule of methane. Covalent bonding is implied inside Lewis structure through indicating electrons contributed between atoms. A covalent bond is a chemical bond which involves the sharing of electron pairs among atoms. These electron pairs are known as shared pairs and the stable balance of attractive and repulsive forces between atoms whenever they share electrons is referred to as covalent bonding.
Covalent Bond