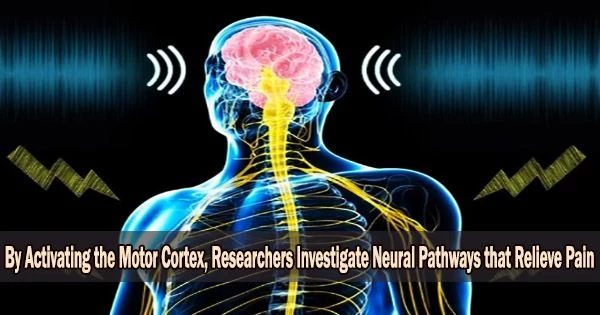
It is possible to study the neural mechanisms underlying pain relief by activating the motor cortex, which is the part of the brain responsible for controlling voluntary movement. This can be done through techniques such as transcranial magnetic stimulation (TMS) or transcranial direct current stimulation (tDCS).
The voluntary contraction of muscles is managed by the motor cortex. The reason why electrical or magnetic stimulation can, albeit unreliable, relieve therapy-resistant chronic pain is still entirely unknown. At the Medical Faculty of Heidelberg (MFHD), an interdisciplinary research team has now identified the underlying mechanisms and neuronal pathways in mice.
The researchers demonstrated how specific motor cerebral cortex nerve pathways process both pain-related information and emotions by directly activating them, which lessens the perception of pain. These pathways also have indirect connections to the brain’s emotion centers.
As a result, the research highlights the brain’s own reward system as a starting point for future treatments in addition to defining a novel brain circuit for neurostimulation in pain therapy. The results are now published in the journal Science.
The research was conducted within the framework of CRC1158 “From Nociception to Chronic Pain,” whose spokesperson is Professor Dr. Rohini Kuner, Director of the Institute of Pharmacology at the MFHD.
We were surprised to find that these neural pathways are indirectly connected to the reward system in deeper brain areas that process emotions. They probably influence the emotional component of the pain experience. This connection was previously unknown and raises interesting new questions.
Professor Dr. Rohini Kuner
If chronic pain, for example resulting from nerve injury (so-called neuropathic pain), does not respond to pharmacological therapies, so-called neurostimulation can be considered. This entails attaching non-invasive electrodes to the scalp and electrically or magnetically stimulating particular cerebral regions.
“There is great need for improvement in this therapy because little is known about how stimulation works, such as where the electrodes should best be placed, which nerve pathways achieve pain relief, and how they respond to stimulation,” explained Prof. Kuner, senior author of the article. “Our findings make an important contribution in explaining the neurobiological basis of brain stimulation for chronic pain and developing it further as a therapeutic procedure.”
The motor cortex, a region of the cerebral cortex whose activation has so far had the greatest impact on chronic pain, was the focus of the researchers’ work. It is made up of a number of layers that are related in various ways.
The first author Zheng Gan, a scientist in Rohini Kuner’s research group, and the team specifically switched on or off the various nerve pathways in this area of the brain using genetic modifications in living mice to test their influence on pain perception.
“We have shown that indeed the motor cortex, more specifically the M1 region, is the key to pain relief. This was previously suspected, but had not been directly demonstrated,” Zheng says.
The scientists also discovered two nerve cell circuits that are crucial to pain alleviation in the two lowest levels of the M1 cortex: These nerve cells, which are located in the penultimate layer 5, affect how the spinal cord processes and perceives pain.
Although their activation lessened the sensitivity to sensory stimuli, persistent discomfort might still be felt. Complete relief didn’t happen until some of the lowest layer 6’s nerve cells were turned on.
“We were surprised to find that these neural pathways are indirectly connected to the reward system in deeper brain areas that process emotions,” Prof. Kuner said. “They probably influence the emotional component of the pain experience. This connection was previously unknown and raises interesting new questions.”
Recommendations for the use of brain stimulation in chronic pain can be derived from the results: It needs to get to the two lowest layers of the motor cortex in order to work best. Additionally, thanks to this work, parameters for therapeutic control may now be generated, which allows for the method’s improvement.
Studies have shown that activating the motor cortex can reduce pain perception in individuals with chronic pain conditions. However, more research is needed to fully understand the mechanisms by which this occurs and to determine the optimal methods for using motor cortex stimulation for pain relief.