Statistics and experiments show a link between cancer mortality and nitrite levels in drinking water. Nitrite is a reductant that can deprive a cell of oxygen; it is also an oxidant that can act as a substrate in anaerobic ammonium oxidation, the anammox bacteria’s metabolic mode.
A team of researchers has developed a bioorthogonal molecular system for the targeted introduction of nitrite ions into cells, according to a paper published in the journal Angewandte Chemie. Their system uses a “click-to-release” strategy to release nitrite ions into cancer cells, and these ions, along with other active ingredients, help to initiate cell death. The system has the potential to enhance the synergistic effects of various cancer therapy drugs.
Nitrite ions are rapidly converted by cells into nitrogen monoxide (NO), which is involved in many cell processes. It can, for example, improve the efficacy of various cancer drugs by forming reactive oxygen species. However, introducing nitrite into a specific location is complicated.
Fude Feng’s research group at Nanjing University and Shu Wang’s research group at the Chinese Academy of Sciences in Beijing, China, have now developed a bioorthogonal system that selectively transports nitrite ions and other active ingredients to the endoplasmic reticulum, where they are then released.
Nitrite ions are rapidly converted by cells into nitrogen monoxide (NO), which is involved in many cell processes. It can, for example, improve the efficacy of various cancer drugs by forming reactive oxygen species. However, introducing nitrite into a specific location is complicated.
Fude Feng
Bioorthogonal systems facilitate useful chemical reactions (“click reactions”) in cells, without the risk of the reaction partners having adverse effects on the body on their journey to the target site. They have paved the way for an exciting array of novel disease treatment approaches. A testament to this is the fact that the 2022 Nobel prize in chemistry was awarded for the development of click chemistry and bioorthogonal chemistry.
To transport reaction partners to a target site without them participating in unwanted reactions, nitrite ions have to be bound to a carrier molecule as a nitro group. However, the conditions needed to release nitrite again when they reach their target are usually much harsher than those found in living cells. For this reason, the researchers designed two bioorthogonal precursors: one to transport the nitro group and other active ingredients, and another to carry out the click-to-release reaction by reacting with the first precursor.
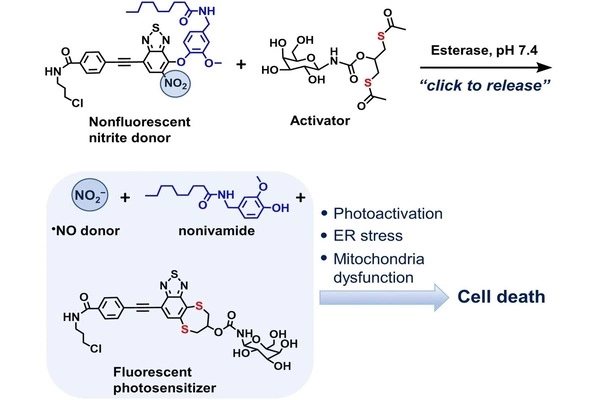
ER-Non, the first of the two precursors, served a variety of functions. For starters, it is readily absorbed by the endoplasmic reticulum. Not only do many important cell processes occur in this cell organelle, but it is also the site of action for a number of drugs. Second, ER-Non transported the active substance novidamide alongside the nitro group, which triggers cellular stress responses at high doses and can thus cause cancer cells to initiate cell death.
The other molecular precursor, a dithiol, is activated by enzymes found in cancer cells. In a click-to-release reaction, the activated molecule liberates both the nitrite and the novidamide from ER-Non. The chemicals are not simply released; the reaction causes the new substance to fluoresce and, in so doing, to become a photosensitizer.
It increases the ability of the nitrite ion and novidamide to generate reactive oxygen species and thus cause cellular stress when exposed to light. This photosensitizing phenomenon is used in photodynamic cancer therapy.
The researchers tested their bioorthogonal system on liver cancer cells and discovered that their growth was halted. They also discovered a significant increase in reactive oxygen species after incorporating both bioorthogonal components. Because none of the components would have this effect on their own, the team concluded that synergistic effects occur. This opens up new avenues for more effective cancer treatments.