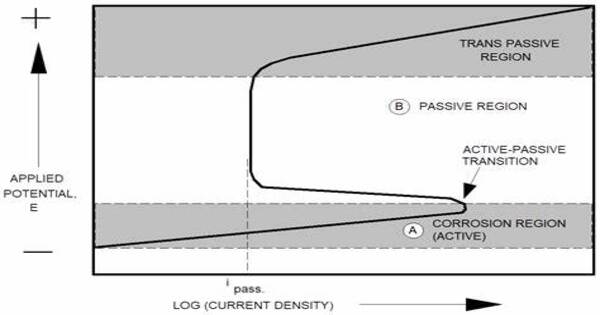
Anodic protection (AP), also known as anodic control, is a technique for preventing corrosion on a metal surface by using it as the anode of an electrochemical cell and adjusting the electrode potential in a zone where the metal is passive. It is a technique for preventing metal corrosion by using it as an anode (positive electrode) in an electrochemical cell. In this procedure, a modest external power source is employed to keep the metal at a potential that allows it to remain passive and resist corrosion.
An anodic protection system consists of an external power supply connected to auxiliary cathodes and controlled by a feedback signal from one or more reference electrodes. Anodic protection requires careful design and control for a variety of reasons, including high current when passivation is lost or unstable, which can result in accelerated corrosion.
Here’s how anodic protection works:
- Electrochemical Cell: It uses an electrochemical cell with the metal to be protected as the anode, a more active metal or an inert electrode as the cathode, and an electrolyte solution.
- Potential Adjustment: The external power source is connected between the metal to be protected (anode) and the cathode. The power source provides a small amount of current to adjust the potential of the metal to a level where it becomes passive and resistant to corrosion.
- Formation of Protective Film: When the metal is maintained at this protective potential, it forms a thin, adherent oxide film on its surface. This film acts as a barrier that prevents further corrosion by isolating the metal from the corrosive environment.
- Monitoring: It’s essential to monitor the current and potential during anodic protection to ensure that the metal remains at the correct protective potential. Changes in current or potential can indicate issues with the protection system or the corrosion environment.
Application
Anodic protection is used to safeguard metals that display passivation in settings where the current density in the freely corroding state is much greater than the current density in the passive state across a wide range of potentials. It is utilized for carbon steel storage tanks holding extreme pH situations such as concentrated sulfuric acid and 50% caustic soda, when cathodic protection is ineffective due to high current requirements.
Advantages
- Localized Protection: Anodic protection can be applied to protect specific areas or localized corrosion sites on a metal surface.
- Cost-effective: It can be more cost-effective than other corrosion protection methods, especially for large structures or equipment.
- Operational Flexibility: Anodic protection can be applied to metals in various environments, including aqueous solutions, soils, and concrete.
Limitations:
- Requires Monitoring: Continuous monitoring is required to maintain the correct protective potential and ensure effective corrosion protection.
- Risk of Overprotection: If the protective potential is set too high, it can lead to hydrogen evolution and embrittlement of the metal.
- Limited Application: Not appropriate for all metals and corrosion conditions. The efficiency of anodic protection is dependent on the individual conditions and kind of corrosion.
Overall, anodic protection is an effective method of avoiding corrosion in select situations when it is appropriate and correctly done. Proper design, monitoring, and maintenance are critical to ensure its effectiveness and durability.