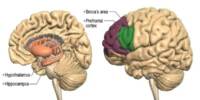
According to research, one night of severe sleep deprivation elevates your levels of beta-amyloid and tau. These are the proteins that have been associated to Alzheimer’s disease. Insomnia also interferes with slow-wave sleep, which is important for learning and memory.
According to a study that also reveals the tau protein is behind that neurodegeneration, the lethargy that many Alzheimer’s patients suffer is caused not by a lack of sleep, but by the loss of a type of neuron that keeps us alert. The study’s findings challenge the widely held belief that Alzheimer’s patients sleep during the day to compensate for a lack of sleep at night, and lead to a new therapy to help these people feel more awake.
The data came from patients at UC San Francisco’s Memory and Aging Center who volunteered to have their sleep monitored with an electroencephalogram (EEG) and to donate their brains after death. The ability to correlate sleep data with microscopic pictures of their post-mortem brain tissue was crucial in answering a question that scientists had been debating for years.
“We were able to prove what our previous research had been pointing to — that in Alzheimer’s patients who need to nap all the time, the disease has damaged the neurons that keep them awake,” said Grinberg, a neuropathologist who, along with psychiatrist Thomas Neylan, MD, is a senior author on the study, which appears in the issue of JAMA Neurology.
“It’s not that these patients are tired during the day because they didn’t sleep at night,” noted Grinberg. “It’s that the system in their brain that would keep them awake is gone.”
We were able to prove what our previous research had been pointing to — that in Alzheimer’s patients who need to nap all the time, the disease has damaged the neurons that keep them awake.
Dr. Grinberg
The reverse phenomenon occurs in patients with other neurodegenerative disorders, such as progressive supranuclear palsy (PSP), who were also included in the study. These individuals have neuronal impairment, which causes them to feel fatigued, and as a result, they are unable to sleep and become sleep deprived.
Grinberg’s team established the theory that Alzheimer’s patients were having difficulty remaining awake after uncovering a set of neurons that keep us awake and are affected in Alzheimer’s from the disease’s inception.
“You can think of this system as a switch with wake-promoting neurons and sleep-promoting neurons, each tied to neurons controlling circadian rhythms,” said Joseph Oh, a medical student and one of the lead authors. “Finally, with this post-mortem tissue, we’ve been able to confirm that this switch, which is known to exist in model animals, also exists in humans and governs our sleep and awake cycles.”
“Extremely Smart Neurons” Disrupted by Tau Proteins
These neurons are “very sophisticated,” according to Oh, because they can create a variety of neurotransmitters and stimulate, inhibit, and control other nerve cells. “It’s a small number of neurons, but their computing powers are amazing,” Oh added. “When these cells are harmed by disease, it can have a significant impact on sleep.”
To figure out what’s causing the degeneration of these neurons in Alzheimer’s, the researchers examined the brains of 33 Alzheimer’s patients, 20 PSP patients, and 32 volunteers who’d had healthy brains until the end of their lives.
The researchers evaluated the levels of two proteins that are frequently related to the neurodegenerative process: beta-amyloid and tau. Which of the two is more important in interrupting sleep has long been debated, with most experts attributing sleep disorders to beta-amyloid buildup.
The brain clears away beta-amyloid that accumulates during the day during sleep. It accumulates when we are unable to sleep. Neylan predicted that because PSP sufferers seldom sleep, she would find a lot of the protein in their brains. “But it turns out they don’t have any,” he explained. “These findings provide direct evidence that tau is a major cause of sleep disruptions.”
In patients with PSP, said Grinberg, this understanding turned the treatment paradigm on its head. “We see that these patients can’t sleep because there is nothing telling the “awake” neurons to shut down,” she said. “Now, rather than trying to induce these people to sleep, the idea is to shut down the system that’s keeping them awake.”
Clinical Trial is Giving Patients Hope
That hypothesis is currently being explored in a clinical trial of PSP patients, who are receiving a drug that directly targets the overactive ‘awake’ system that prevents these patients from sleeping. This method differs from the typical trial-and-error treatment with sleep medicines.
Christine Walsh, Ph.D., the study’s other principal author, who has also worked on the topic for a decade, is in charge of that trial. She expects the findings will lead to new ways of treating sleep disruptions caused by neurodegeneration, despite the fact that PSP and Alzheimer’s are at opposing extremities of the sleep-disorder spectrum.
Treatments for Alzheimer’s disease might be tailored to the patient’s demands, increasing the “awake” system while decreasing the “sleep” system, according to Walsh, who, along with Grinberg, is a member of the UCSF Weill Institute for Neurosciences.
The PSP experiment is currently ongoing, and Walsh is confident that this novel technique will produce better results than current drugs for those suffering from either illness. “We’re even more hopeful that we can truly make a difference in the lives of these individuals,” she added, based on the study’s findings.