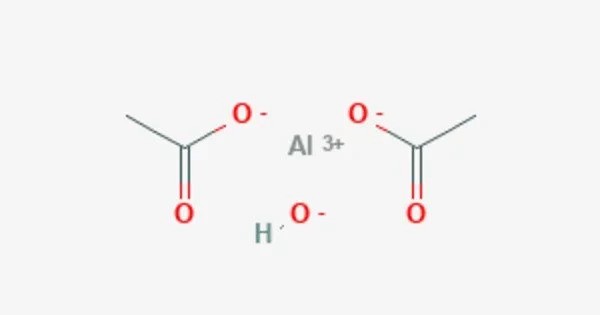
Aluminium triacetate, formally named aluminium acetate, is a chemical compound with composition Al(CH3CO2)3. It appears as a white, crystalline solid and is moderately soluble in water, forming a slightly acidic solution due to hydrolysis. Under standard conditions it appears as a white, water-soluble solid that decomposes on heating at around 200 °C. The triacetate hydrolyses to a mixture of basic hydroxide / acetate salts, and multiple species co-exist in chemical equilibrium, particularly in aqueous solutions of the acetate ion; the name aluminium acetate is commonly used for this mixed system.
It is commonly used in medicine, particularly as an astringent and antiseptic in topical treatments like Burow’s solution for skin conditions such as eczema, insect bites, or rashes. Its astringent properties help reduce inflammation and dry out affected areas.
Properties
- Chemical formula: C6H9AlO6
- Molar mass: 204.114 g·mol−1
- Appearance: white solid
- Solubility in water: soluble
Natural Occurrence
Aluminium triacetate is not commonly found in nature as a distinct mineral or compound due to its tendency to hydrolyze in aqueous environments. However, aluminium ions and acetate groups can coexist in natural systems (e.g., soils or water bodies with organic acids), potentially forming transient complexes. It may occur in trace amounts in environments rich in aluminium-containing minerals (e.g., bauxite) and organic matter that produces acetic acid.
Synthesize
Aluminium triacetate is synthesized by reacting aluminium hydroxide or aluminium sulfate with acetic acid. The reaction yields the triacetate form, though basic aluminium acetates, like Al(OH)(C₂H₃O₂)₂ or Al(OH)₂(C₂H₃O₂), may form under specific conditions. It is stable under normal conditions but decomposes upon heating, releasing acetic acid vapors.
Applications
In industrial applications, it serves as a mordant in textile dyeing, enhancing color adhesion to fabrics. It is also used in antiperspirants due to its ability to constrict sweat glands. While generally safe for topical use, excessive exposure may cause skin irritation. Its environmental impact is minimal, as it degrades into non-toxic compounds. Proper handling is advised to avoid inhalation or ingestion.
- Medical Use: Aluminium acetate (often as a solution like Burow’s solution) is used as an astringent and antiseptic for skin conditions such as eczema, insect bites, or rashes. It reduces inflammation and promotes drying of wet or oozing skin lesions.
- Textile Industry: Employed as a mordant in dyeing processes to fix dyes onto fabrics by forming complexes with dye molecules.
- Catalysis: Used in some catalytic processes due to aluminium’s ability to form coordination complexes.
- Material Science: Acts as a precursor for aluminium-based ceramics or coatings.
- Analytical Chemistry: Utilized in the preparation of reagents for chemical analysis.