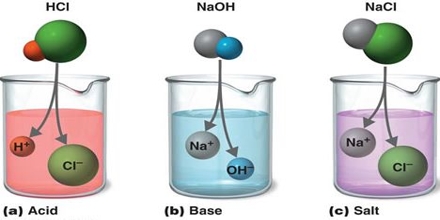
Major objective of this lecture is to describe on Acids, Bases and Salts. Acids are substances that form hydrogen ions, when dissolved in water. Bases are oxides and hydroxides of metals that react and neutralize acids to form salts and water only. Bases can be strong or weak depending on the extent to which they dissociate and produce OH– ions in solution. Here also briefly describe on common strong and weak acids and their anions. Finally focus on Physical Properties of Acids & Bases.
Acids, Bases and Salts