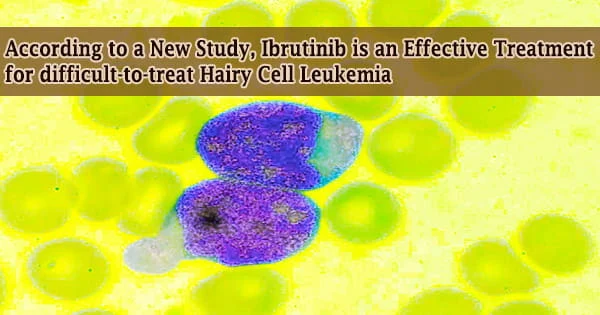
According to a new study conducted by experts at The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute (OSUCCC James), the oral targeted therapy drug ibrutinib is an effective therapeutic choice for high-risk hairy cell leukemia.
Ibrutinib, also known as Imbruvica, is a small molecule medication that inhibits B-cell proliferation and survival by binding to the protein Bruton’s tyrosine kinase (BTK) in an irreversible manner. The B-cell receptor pathway, which is often abnormally active in B cell malignancies, is inhibited by blocking BTK. Ibrutinib is available as a capsule or a tablet for oral use. It’s typically taken once a day.
Ibrutinib should be taken at the same time every day. Follow the directions on your prescription label carefully, and if there is anything you don’t understand, ask your doctor or pharmacist to explain it to you. Ibrutinib should be taken exactly as prescribed. Do not take more or less of it, or take it more frequently than your doctor has suggested.
Hairy cell leukemia is an uncommon type of B-cell blood cancer that affects about 600 to 800 persons in the United States every year. It is more frequent in middle-aged and older persons, and it affects more men than women. Ibrutinib oral bioavailability is 3.9 percent when fasting, 8.4 percent when fed, and 15.9 percent when grapefruit juice is consumed.
Hairy cell leukemia is classified as a chronic disease since it is unlikely to go away completely, despite the fact that treatment can result in years of remission. While the disease has a fair prognosis for the majority of people who are affected, a tiny minority of patients with variants of the disease do not respond well to existing FDA-approved medications or cannot endure the side effects of existing therapies, according to the researchers.
This is an effective, well-tolerated new treatment option for patients impacted by the highest-risk forms of hairy cell leukemia. It’s a very exciting development that could transform survivorship for this subset of patients from months and years to years and decades.
Dr. Kerry Rogers
“There is a critical unmet need for therapy options in this subset of patients to achieve long-term cancer control,” said Dr. Kerry Rogers, principal investigator of the clinical trial and a hematologist/scientist at the OSUCCC James.
“Our study shows that ibrutinib (pronounced eye-broo-ti-nib) is a safe, effective, and well-tolerated option for patients with relapsed or variant forms of hairy cell leukemia. It is a very important discovery for patients facing this diagnosis.”
A multi-institutional team led by the OSUCCC James recruited 44 patients with high-risk hairy cell leukemia for this phase 2 clinical research, 15 of whom were treated at the OSUCCC James in Columbus, Ohio.
All of the participants in the study had either classic hairy cell leukemia and had previously received other treatments, or a variant form of the disease in which the standard therapies of cladribine (pronounced KLAD-rih-been) and pentostatin (pronounced PEN-toh-STA-tin) are unlikely to be effective.
Researchers reported their findings in the June 24 issue of Blood.
Ibrutinib’s efficacy has been reported as a quick reduction in lymphadenopathy accompanied by a transitory lymphocytosis in early clinical investigations, suggesting that the drug may have direct effects on cell homing or migration to components in tissue microenvironments.
Ibrutinib is an oral treatment that belongs to the Bruton’s tyrosine kinase (BTK) inhibitor class of medicines. These medications inhibit biological functions by blocking particular chemical reactions in the body.
The medicine was used in this trial as an experiment, however, it is approved by the FDA for the treatment of diseases such as mantle cell lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma, and others.
“The underlying cellular biology of these diseases is similar, so we wanted to determine if this FDA-approved drug that is used to treat other forms of blood cancer could also serve as an effective treatment for this small segment of hairy cell leukemia patients who did not respond to traditional therapies,” said Rogers, who is an assistant professor in Ohio State’s College of Medicine.
“Even though hairy cell leukemia is a disease with a generally good prognosis, there is a small group of patients for whom current therapies are inadequate for cancer control,” Rogers added.
“This is an effective, well-tolerated new treatment option for patients impacted by the highest-risk forms of hairy cell leukemia. It’s a very exciting development that could transform survivorship for this subset of patients from months and years to years and decades.”
Keep this medication tightly wrapped in the container it came in and out of the reach of children. It should be stored at room temperature, away from light, heat, and moisture, and not in the bathroom.
Because many containers (such as weekly pill minders and those for eye drops, creams, patches, and inhalers) are not child-resistant and small children can readily open them, it is critical to keep all medication out of sight and reach of children.
This study was sponsored by the Cancer Therapy Evaluation Program at the National Cancer Institute and grants from the National Cancer Institute/National Institutes of Health and conducted at the OSUCCC James; the NCI clinical trials center, Karmanos; Mayo Clinic and MD Anderson Cancer Center. The study began in 2013 and is closed to patient accrual.