About Antibodies
Definition
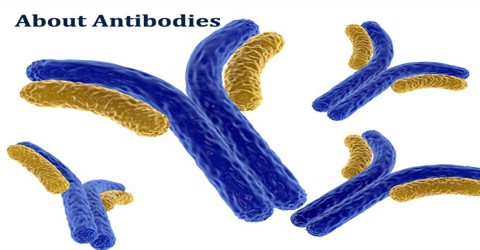
Antibodies, also known as immunoglobulins, are Y-shaped proteins that are produced by the immune system to help stop intruders from harming the body. When an intruder enters the body, the immune system springs into action. These invaders, which are called antigens, can be viruses, bacteria, or other chemicals. When an antigen is found in the body, the immune system will create antibodies to mark the antigen for the body to destroy.
Antibodies are secreted by B cells of the adaptive immune system, mostly by differentiated B cells called plasma cells. Antibodies can occur in two physical forms, a soluble form that is secreted from the cell to be free in the blood plasma, and a membrane-bound form that is attached to the surface of a B cell and is referred to as the B-cell receptor (BCR). The BCR is found only on the surface of B cells and facilitates the activation of these cells and their subsequent differentiation into either antibody factories called plasma cells or memory B cells that will survive in the body and remember that same antigen so the B cells can respond faster upon future exposure.
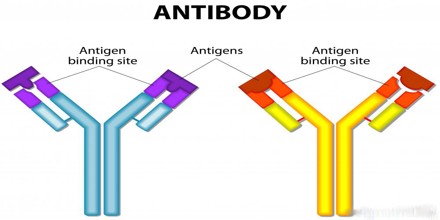
The antibodies act sort of like the immune system’s scouts. They find antigens, stick to them, and identify for the immune system the exact type of antigen so that it can be destroyed. Each antibody is made for one and only one antigen, and it’s fitted with special receptors that will only bind to that antigen. For instance, a specific antibody is created to help destroy the chickenpox virus. Only that particular antibody will attack a chickenpox virus.
Immunizations take advantage of the fact that antibodies remain in the body after an infection is eradicated. Most immunizations consist of a weak or diluted form of an antigen – not enough of the antigen to make the patient sick, but just enough to trigger the creation of antibodies. This way, the body can instantly attack any form of the infection it encounters, stopping the infections before they begin.
Structure of Antibodies
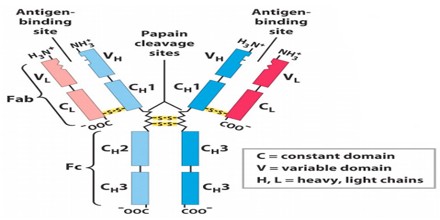
Antibodies all have the same basic structure consisting of two heavy and two light chains forming two Fab arms containing identical domains at either end attached by a flexible hinge region to the stem of the antibody, the Fc domain, giving the classical ‘Y’ shape.
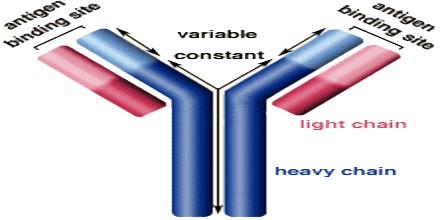
The constant region determines the mechanism used to destroy antigen. Antibodies are divided into five major classes, IgM, IgG, Iga, IgD, and IgE, based on their constant region structure and immune function.
The variable region is further subdivided into hypervariable (HV) and framework (FR) regions. Hypervariable regions have a high ratio of different amino acids in a given position, relative to the most common amino acid in that position. Within light and heavy chains, three hypervariable regions exist – HV 1, 2 and 3. Four FR regions which have more stable amino acids sequences separate the HV regions.
The HV regions directly contact a portion of the antigen’s surface. For this reason, HV regions are also sometimes referred to as complementarity determining regions, or CDRs. The FR regions form a beta-sheet structure which serves as a scaffold to hold the HV regions in position to contact antigen.
Function of Antibodies
The two structural portions of the antibody, i.e. the variable (Fab) and the constant (Fc) fragments, impart distinct biological functions. These functions are outlined as follows:
Fab-Mediated Functions:
- Antigen recognition – One of the major functions of the Fab region is antigen recognition. The immune system generates large number of antibodies that can recognize virtually all possible antigens present in pathogens and their products. Antibodies can be produced against all types of molecules including carbohydrates, nucleic acids and phospholipids but are best suited to bind against a protein.
- Neutralization of pathogens – Once the antibodies recognize the antigens the binding occurs outside the cell. This is where most of the bacteria and bacterial toxins are found. Antibodies also block the binding of the bacteria to host cells by binding to cell-surface proteins. Antibodies protect similarly from viral infections as well.
- Antibodies are the first line of defence – IgM antibodies have a pentameric structure and are rapidly generated in blood. They can bind to multivalent antigens, such as bacterial cell wall polysaccharides.
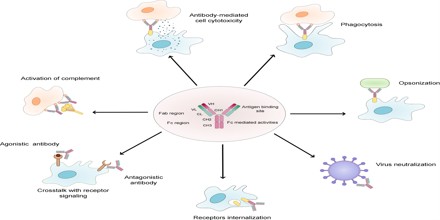
Fc-Mediated Effector Functions:
- Activation of effector cells – These include phagocytic cells like macrophages and neutrophils, T cells like natural killer cells, and eosinophils and mast cells. Each of these cells has a receptor for the Fc fragment. Thus these cells can identify an Fc fragment and eliminate the pathogens.
- Complement binding – Once bound to the antigen there is formation of antigen–antibody complexes. The main functions of the complements are to enable phagocytes to destroy bacteria that they would otherwise not recognize. Neither complement nor phagocytes are specific for the pathogen but the antibodies are.
- Opsonization – Those microbes that replicate outside cells are removed by an interaction of the Fc part with specific receptors on the surface of elector cells. The antibodies coat the surface of the pathogen and allow binding of their Fc domains to Fc receptors present on elector cells.