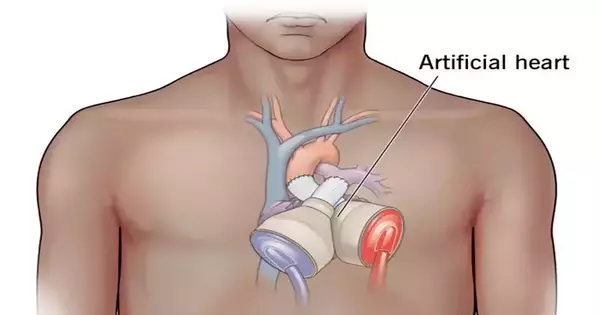
Bioengineers created the first biohybrid model of human ventricles with helically aligned beating cardiac cells, demonstrating that muscle alignment does, in fact, increase the amount of blood the ventricle can pump with each contraction.
Heart disease is the leading cause of death in the United States, in part because the heart, unlike other organs, cannot repair itself after injury. That is why tissue engineering, eventually including the wholesale fabrication of an entire human heart for transplant, is critical for the future of cardiac medicine.
Researchers must replicate the unique structures that make up the heart in order to build a human heart from the ground up. This includes recreating helical geometries, which cause the heart to twist as it beats. It’s long been assumed that this twisting motion is essential for pumping blood at high volumes, but proving it has been difficult, thanks in part to the difficulty of creating hearts with different geometries and alignments.
Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS) bioengineers have created the first biohybrid model of human ventricles with helically aligned beating cardiac cells, demonstrating that muscle alignment does, in fact, dramatically increase the amount of blood the ventricle can pump with each contraction.
This advancement was made possible using a new method of additive textile manufacturing, Focused Rotary Jet Spinning (FRJS), which enabled the high-throughput fabrication of helically aligned fibers with diameters ranging from several micrometers to hundreds of nanometers. Developed at SEAS by Kit Parker’s Disease Biophysics Group, FRJS fibers direct cell alignment, allowing for the formation of controlled tissue engineered structures.
The research is published in Science.
The human heart actually has multiple layers of helically aligned muscles with different angles of alignment. With FRJS, we can precisely recreate those complex structures, forming single and even four chambered ventricle structures.
Huibin Chang
“This work is a significant step forward for organ biofabrication, bringing us closer to our ultimate goal of building a human heart for transplant,” said Parker, the Tarr Family Professor of Bioengineering and Applied Physics at SEAS and the paper’s senior author.
This work is based on a centuries-old mystery. In his seminal work Tractatus de Corde, English physician Richard Lower, who counted John Locke among his colleagues and King Charles II among his patients, first noted the spiral-like arrangement of heart muscles in 1669.
Over the next three centuries, physicians and scientists gained a more complete understanding of the heart’s structure, but the function of those spiraling muscles remained frustratingly difficult to investigate.
Edward Sallin, former chair of the Department of Biomathematics at the University of Alabama Birmingham Medical School, argued in 1969 that the helical alignment of the heart is critical to achieving high ejection fractions (the percentage of blood the ventricle pumps with each contraction).
“Our goal was to build a model where we could test Sallin’s hypothesis and study the relative importance of the heart’s helical structure,” said John Zimmerman, a postdoctoral fellow at SEAS and co-first author of the paper.
To test Sallin’s theory, the SEAS researchers used the FRJS system to control the alignment of spun fibers on which they could grow cardiac cells.
The first step of FRJS works like a cotton candy machine: a liquid polymer solution is loaded into a reservoir and centrifugal force pushes it out through a tiny opening as the device spins. The solvent evaporates as the solution exits the reservoir, and the polymers solidify to form fibers. The orientation of the fiber is then controlled by a focused airstream as it is deposited on a collector. The researchers discovered that by angling and rotating the collector, the fibers in the stream aligned and twisted around it as it spun, mimicking the helical structure of heart muscles.
The alignment of the fibers can be tuned by changing the angle of the collector.
“The human heart actually has multiple layers of helically aligned muscles with different angles of alignment,” explained Huibin Chang, a postdoctoral fellow at SEAS and the paper’s co-first author. “With FRJS, we can precisely recreate those complex structures, forming single and even four chambered ventricle structures.”
Unlike 3D printing, which slows down as features become smaller, FRJS can rapidly spin fibers at the single micron scale – roughly fifty times smaller than a single human hair. This is critical when constructing a heart from the ground up. Consider collagen, an extracellular matrix protein found in the heart that is also a single micron in diameter. It would take more than 100 years to 3D print every bit of collagen in the human heart at this resolution. FRJS can do it in a single day.
The ventricles were seeded with rat cardiomyocyte or human stem cell derived cardiomyocyte cells after spinning. Within a week, several thin layers of beating tissue had covered the scaffold, with the cells aligning with the fibers beneath.
The beating ventricles resembled the twisting or wringing motion of human hearts.
The researchers compared the deformation of the ventricle, the speed of electrical signaling, and the ejection fraction of ventricles made of helical aligned fibers and those made of circumferentially aligned fibers. They discovered that helically aligned tissue outperformed circumferentially aligned tissue on all fronts.