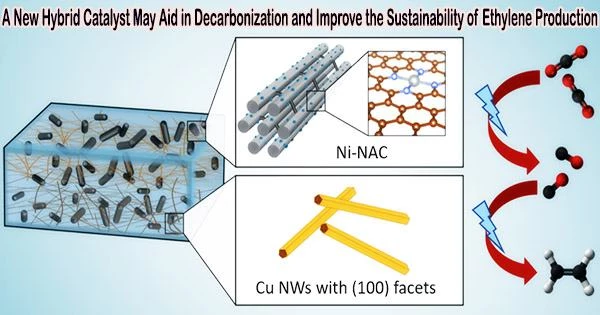
Carbon dioxide is converted into ethylene in one step using a novel hybrid catalyst. Scientists from Ames National Laboratory, Iowa State University, University of Virginia, and Columbia University developed the catalyst. By utilizing carbon dioxide (CO2) as a fuel for effective ethylene production powered by electricity, this catalyst helps the global net-zero carbon endeavor.
A common molecule called ethylene is used to make a variety of goods, including plastics and antifreeze. Ethylene is produced on a huge scale, which requires a lot of energy and fossil fuels.
Electrocatalytic production of ethylene from CO2 is emerging as a promising method. Only earth-abundant components, including nickel and copper, make up this new catalyst, which uses less energy during chemical reactions.
Long Qi, a scientist at Ames Lab, explained how the catalyst works. At low voltage and high current, atomically distributed nickel anchored on nitrogen assembly carbon (NAC) catalyzes the conversion of CO2 to CO. The catalyst works well at a variety of voltages, and it produces more CO at greater currents due to its effectiveness.
And we can do this without any precious metal, simply the nickel, copper, carbon, and nitrogen, to permit large-scale industrial applications. Also, we potentially eliminate the use of fossil resources to make ethylene.
Long Qi
“Since this catalyst remains active over a very wide voltage range, that allows easy coupling with a second catalyst,” Qi said. “So we use the second catalyst, which is a copper nanowire, and by combining these two we have a very selective process that has up to 60% efficiency going from CO2 to ethylene in one pot.”
Another important aspect of the catalyst is its structure. Wenyu Huang, an Ames Lab scientist and Iowa State University professor from the team, noted that the catalyst’s porous structure enhances its effectiveness.
“Our catalyst has an ordered mesoporous structure that is beneficiary for mass transfer,” he said. “Because it’s highly porous, you have a very high surface area to expose a lot of nickel’s active sites, making our catalyst very effective in CO2 reduction to CO.”
The way the researchers blended the two catalysts to speed up the process was Huang’s favorite part of the research.
“We basically combine the two best catalysts on their own, and they work together so we can connect the CO2 to CO and the CO to ethylene reactions in one system,” he said.
Because it addresses the need to reduce CO2 emissions globally, Qi stressed the significance of employing CO2 as a feedstock for this process. He clarified that CO2 recovered through industrial or chemical operations, as well as from air capture, can be used in this procedure.
“And we can do this without any precious metal, simply the nickel, copper, carbon, and nitrogen, to permit large-scale industrial applications,” Qi said. “Also, we potentially eliminate the use of fossil resources to make ethylene.”