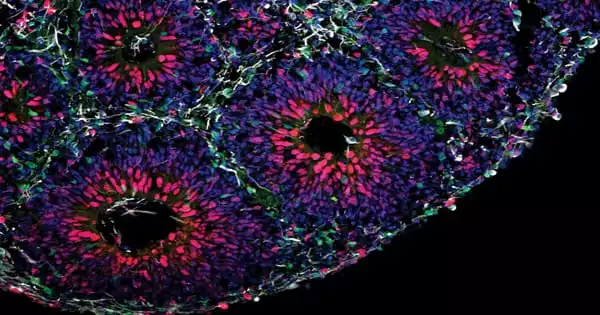
Brain organoids are used by scientists to study how a faulty gene impacts brain development. It is critical to look at what happens in the brain throughout development to better understand the origins of autism spectrum disorders (ASD). The closest we’ve got to viewing human brains this early is through the use of organoids, which are microscopic models of organs. Scientists at the Institute of Science and Technology Austria (ISTA) used their assistance to determine how mutations in a high-risk gene for autism disrupt crucial developmental processes.
Autism spectrum illnesses are linked to hundreds of genes. Some patients are just minimally afflicted, whereas others are severely disabled. In addition to typical symptoms such as difficulties in social interaction and communicating with others, as well as repetitive-stereotypic behaviors, patients with CHD8 gene mutations frequently exhibit intellectual deficiencies and macrocephaly (an exceptionally big brain). It has long been unknown how CHD8 generates these symptoms.
Tiny artificial brains
Since CHD8 mutations affect the brain at a very early stage of its development, it has proven difficult for scientists to get the full picture. Over the past years, many researchers, therefore, used mice as model organisms to better understand what is going on. “But mice with a CHD8 mutation barely showed the symptoms human patients are showing. The effects in mice are not comparable to humans. We needed some kind of human model,” Professor Gaia Novarino explains.
Our understanding of how varied paths affect brain functions remains inadequate.” The fundamentals of brain development must be better understood in order to one day aid patients with a CHD8 mutation.
Bárbara Oliveira
Novarino and her team at ISTA resorted to organoids in collaboration with collaborators from the Italian Human Technopole institute, the European Institute of Oncology, and the University of Milan, as well as the Allen Institute for Brain Science in the United States. These simplistic tiny organs are generated from stem cells, which have the power to transform into nearly any other sort of cell. The scientists were able to construct basic forms of brain tissue the size of lentils by generating the appropriate conditions and providing the right input at the right moment.
“Organoids are the only way you can study human brain development at such an early phase,” says Bárbara Oliveira, postdoc in the Novarino group and one of the authors of the study.
CHD8 mutations disrupt balance of neuron production
In petri dishes the team created brain organoids with and without mutations of the gene CHD8. “After some time, we could see by eye that the mutant organoids were much bigger. That was the first evidence that the model works,” her colleague and co-author, Ph.D. student Christoph Dotter, describes. Like patients with a CHD8 mutation, the organoids were showing signs of brain overgrowth.
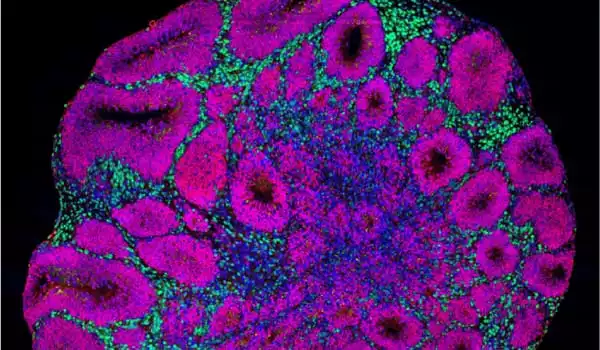
As the crew examines all of the cell types in the organoids, they find something very early on: Much earlier than the control group, the mutant organoids began to develop a specific type of neuron, inhibitory neurons. Excitatory neurons, on the other hand, were created later. Furthermore, the mutant organoids produced significantly more proliferating cells, which later created a greater number of this type of neuron. Overall, the authors found, that this results in them being much larger than organoids lacking CHD8 mutations, which correlate with patients’ macrocephaly.
Starting to understand our brain
The Novarino group’s most recent study, like earlier ones, demonstrates the importance of time in autism research. “Looking at multiple time points provides us knowledge that what you see in the end might not be the complete picture of how a patient’s brain developed — much more could have happened previously,” adds Novarino. “Our understanding of how varied paths affect brain functions remains inadequate.” The fundamentals of brain development must be better understood in order to one-day aid patients with a CHD8 mutation. The Novarino group was able to make a significant contribution by recreating genetic and clinical traits from ASD patients in brain organoids.
“Genetic studies have been extraordinarily effective in discovering abnormalities in the genome associated with autism spectrum disorders and other neurodevelopmental diseases.” “The difficult next step on the path to discovering new treatments is to understand exactly what these mutations do to the developing brain,” said Steven Hyman, a Harvard University Distinguished Service Professor of Stem Cell and Regenerative Biology, the director of the Stanley Center at the Broad, and a Broad Institute core member. “By identifying the changes in brain circuits that occur when genetic variants are present, we can take a tentative next step toward better diagnosis and find new pathways for therapeutic research.”