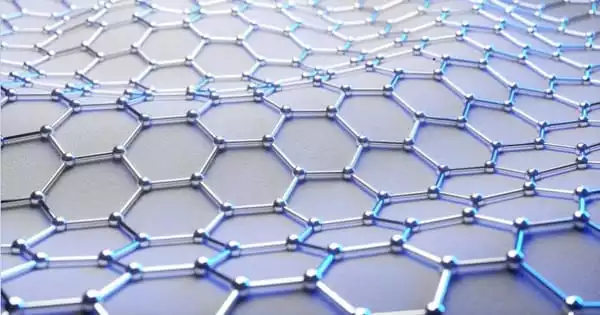
Holey graphyne is a new kind of carbon allotrope with semiconductor properties that can be used in photoelectronics, sensors, and water purification. Carbon has various faces, including graphene, nanotubes, fullerene, and graphdiyne, all of which are made up primarily of hexagons.
Diamond and graphite have been recognized for thousands of years as two naturally occurring carbon allotropes. They are elemental carbons that are organized in such a way that they contain sp3 and sp2 hybridized carbon atoms. The discovery of other carbon allotrope materials, such as graphene, fullerene, carbon nanotube, graphyne, and graphdiyne, has recently revolutionized modern nanomaterials science. Because of its interesting features, graphene research has achieved substantial discoveries in modern chemistry and physics.
Carbon allotrope materials like graphene, carbon nanotubes, fullerene, graphyne, and graphdiyne were recently found, transforming modern nanomaterials science. Graphene research has achieved important advances in modern chemistry and physics because to its appealing features. Graphene has been heralded as a miracle material with the potential to change the semiconductor industry due to its exceptional electron mobility capabilities. Despite the hoopla, there is still a long way to go before we reach the graphene age.
Two aromatic benzene rings are linked by two bent acetylenic links in dibenzocyclooctadiyne, resulting in a highly strained eight-membered ring. This fascinating chemical led us to create and synthesize a new carbon allotrope, namely holey-graphyne.
Associate Director Lee Hyoyoung
Because of its outstanding electron mobility qualities, graphene has been heralded as a miracle material with the potential to revolutionize the semiconductor industry. Despite the excitement, it appears that our civilization is still a long way from shifting from the silicon age to the graphene age. The fundamental issue of employing graphene in electronics is its zero-bandgap electrical structure. This makes it hard to turn off graphene-based transistors, limiting its application in the semiconductor sector. While it is feasible to overcome this constraint by doping or functionalizing graphene, there is also great interest in the quest for new types of 2D carbon allotropes with extraordinary semiconducting properties, such as a correct energy bandgap and high mobility.
Researchers have discovered that by producing many holes in the structure of graphene or graphene oxides, it is feasible to give many features required for a semiconductor. This novel substance is known as “holey graphene.” In comparison to graphene, -graphyne, or graphdiyne, holey graphene not only has ideal 2D semiconducting properties, but it also has nonlinear sp bonding and a special -conjugated structure, which offers promising applications in optoelectronic, energy harvesting, gas separation, catalysis, water remediation, sensor, and energy-related fields.
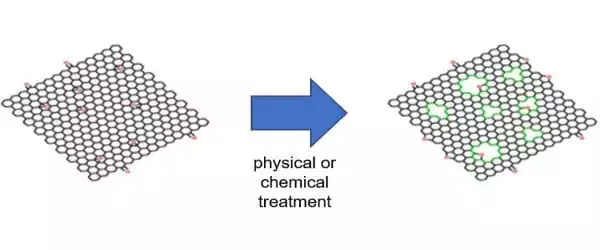
So far, holey graphene has been created in laboratories by first synthesizing graphene and then subjecting the graphene to physical, chemical, or hydrothermal treatment to puncture many holes in the structure. However, such a top-down approach for production has its limitations because the size and distribution of the ‘holes’ are uneven and difficult to control.
Researchers from South Korea’s Institute for Basic Science’s Center for Integrated Nanostructure Physics (CINAP), led by Associate Director LEE Hyoyoung, established a bottom-up strategy for manufacturing such material. The team developed a way for building topologically 2D carbon material atom by atom for the first time.
The scientists termed this new two-dimensional single-crystalline material “holey-graphyne” (HGY). HGY is formed of a pattern of six-vertex and highly strained eight-vertex rings, as well as an equal amount of sp2 and sp hybridized carbon atoms that are alternately bonded between benzene rings and C≡C bonds.
“We were motivated by the fascinating chemical dibenzocyclooctadiyne, which was initially synthesized in 1974 by Sondheimer and colleagues. Two aromatic benzene rings are linked by two bent acetylenic links in dibenzocyclooctadiyne, resulting in a highly strained eight-membered ring. This fascinating chemical led us to create and synthesize a new carbon allotrope, namely holey-graphyne” Associate Director Lee stated.
The ultra-thin single-crystalline HGY was successfully manufactured by the study team utilizing 1,3,5-tribromo-2,4,6-triethynylbenzene as the basic material. The single atomic layer thin HGY was then created by combining the interfaces of two solvent systems, water and dichloromethane. The novel HGY has a direct bandgap of around 1.1 eV and good calculated-carrier mobility, making it suitable for use as a semiconductor material.
This new finding not only shows the first synthesis of ultrathin single crystalline HGY, but it also presents a new approach for the design and synthesis of such a novel type of 2D carbon allotrope. The future application of HGY in the semiconductor industry is expected to pave the way for a new generation of devices beyond the silicon age.