The term “prions” refers to abnormal, pathogenic agents that are transmissible and capable of inducing abnormal folding of specific normal cellular proteins known as prion proteins, which are abundant in the brain. These normal prion proteins’ functions are still poorly understood.
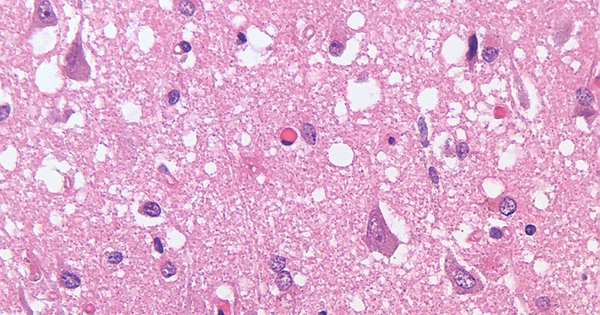
Neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and Huntington’s disease are characterized by the deposition of protein clumps in patients’ brains, known as protein aggregates. Although disease-relevant proteins, such as the huntingtin protein in Huntington’s disease, are present in all cells of the human brain, huntingtin aggregates form in a specific region of the brain during the early stages of the disease.
A recent study by the group of Ulrich Hartl from the Max Planck Institute of Biochemistry investigates the influence, that the cell type has on this preference of aggregate formation in a distinct brain region. The study has been published in the scientific journal Molecular Cell. To address this phenomenon, the researchers performed experiments in a yeast model system.
Neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and Huntington’s disease are characterized by the deposition of protein clumps in patients’ brains, known as protein aggregates.
Artificial protein aggregation through blue light illumination
Similar to the human brain, the formation of huntingtin aggregates in yeast also depend on the cell type, the so-called yeast strain. While the huntingtin protein forms aggregates in some yeast strains, it remains soluble in others. Why this is the case hasn’t been understood so far.
To investigate the distinction between different yeast strains and how they contribute to the formation of huntingtin aggregates, the researchers utilized recent advances in the field of optogenetics. They biotechnologically manipulated yeast strains that normally do not allow the aggregation of huntingtin and integrated a molecular switch that could be activated with blue light. This way, huntingtin aggregates could be formed simply by illuminating the cells with blue light.
Comparing the yeast cells that naturally form huntingtin aggregates with those, that only do so after their activation with blue light, caught the researchers by surprise. Only in cells where huntingtin aggregates already form naturally, but not in those where the aggregation of huntingtin was artificially induced with blue light, were toxic effects observed.
The first author of the study, Michael Gropp, reasoned that this phenomenon came about because smaller intermediates, rather than large aggregates, are the actual toxic version of the protein. Only in yeast cells that form huntingtin aggregates naturally, do these smaller toxic intermediates, the oligomers, exist. Here, large aggregates arise slowly, through the accumulation of proteins around the smaller intermediates.
These small intermediates are bypassed when the aggregation of huntingtin is induced artificially with blue light. Large aggregates then appear much more rapidly, avoiding toxic effects.
The role of prions during aggregate formation
But why do some yeast strains form huntingtin aggregates, while other genetically identical strains do not? Further assays in yeast and experiments with purified proteins—proteins that were artificially enriched in a test tube—helped the researchers to understand this phenomenon. Some yeast strains naturally contain protein aggregates of certain proteins, the prions.
These prion aggregates are not harmful for the cells. However, due to their specific structure, these prion aggregates can influence soluble huntingtin proteins and impose their structure on them. As a result, soluble huntingtin proteins convert into an aggregated state. A side effect of this process is the appearance of toxic intermediates. Yeast strains that naturally do not form huntingtin aggregates also do not possess prions and are therefore unable to generate toxic intermediates, despite the artificial induction of large huntingtin aggregates with blue light.
Possible implications for human disease
In recent years, many human proteins have been characterized that share similarities with the prions in yeast. A bioinformatic analysis of previously published data sets from mouse models and human cell cultures showed that mammalian proteins with such prion-like characteristics preferentially accumulate in neurons.
With the increasing age of an individual, they tend to form aggregates. The authors of the study suspect that the aggregates of these prion-like proteins can in turn force the aggregation of disease-relevant proteins, such as huntingtin, in certain brain areas and thus contribute to the disease progression in neurodegenerative disorders. Further investigation of this hypothesis is still ongoing.