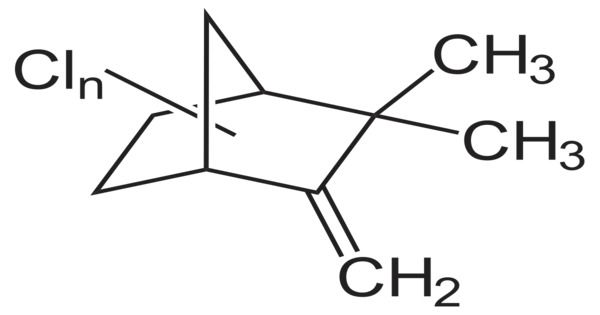
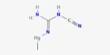
Toxaphene was an insecticide that was predominantly employed on cotton in the southern United States throughout the late 1960s and 1970s. Toxaphene is a compound composed of about 670 distinct compounds that are formed by reacting chlorine gas with camphene. It is most usually seen as a waxy solid ranging from yellow to amber. It is a complex blend of about 670 different compounds, making it one of the most complicated insecticides ever developed.
Toxaphene is a synthetic chemical molecule commonly used as a pesticide. It was first launched in the 1940s and was widely used to control pests in agriculture, as well as insects in buildings and cattle. It was banned in the United States in 1990 and globally in 2001 by the Stockholm Convention on Persistent Organic Pollutants. It is a highly persistent chemical that can survive in the environment for 1-14 years without decomposing, especially in soil.
However, toxaphene has been identified as an extremely dangerous and long-lasting environmental pollutant. It has been shown to bioaccumulate in the food chain, notably in animal fatty tissues, and to remain in the environment for extended periods of time. Due to these concerns, its use has been heavily restricted or banned in many countries.
Animal testing, primarily on rats and mice, has shown that toxaphene is toxic to them. Toxaphene has been shown to activate the central nervous system while also inducing morphological changes in the thyroid, liver, and kidney. Its exposure has been related to a variety of human health problems, including neurological, reproductive, and developmental disorders. It is also considered a possible carcinogen.
Because of its tenacity and capacity to move great distances via air and water currents, toxaphene pollution has been discovered in isolated places far from its initial application sites. activities to reduce its environmental impact include cleanup and remediation activities, as well as tight usage and disposal rules.