A breakthrough could pave the way for new HIV treatments while also shedding light on the role of the innate immune system in other diseases. Human immunodeficiency virus 1, also known as HIV-1, is notorious for its uncanny ability to evade the immune system. Scripps Research and collaborators have now discovered how our innate immune system detects HIV-1, even when it is present in trace amounts.
The findings, published in the journal Molecular Cell, reveal a two-step molecular strategy that activates the innate immune response when exposed to HIV-1. This discovery has the potential to influence drug development for HIV treatments and vaccines, as well as our understanding of how the innate immune response is involved in other areas, such as neurodegenerative disorders like Alzheimer’s.
“This research delineates how the immune system can recognize a very cryptic virus, and then activate the downstream cascade that leads to immunological activation,” says Sumit Chanda, PhD, professor in the Department of Immunology and Microbiology. “From a therapeutic potential perspective, these findings open up new avenues for vaccines and adjuvants that mimic the immune response and offer additional solutions for preventing HIV infection.”
While the adaptive immune system has been a main focus for HIV research and vaccine development, our discoveries clearly show the critical role the innate immune response plays in detecting the virus. This research delineates how the immune system can recognize a very cryptic virus, and then activate the downstream cascade that leads to immunological activation.
Sunnie Yoh
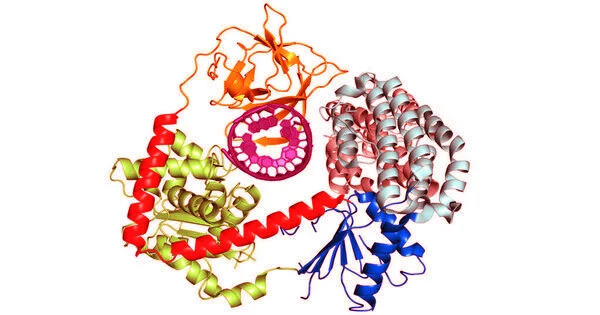
The innate immune system is activated first, followed by the adaptive immune system, which is the body’s secondary line of defense and performs more specialized functions such as antibody production. One of the primary functions of the innate immune system is to distinguish between “self” (our own proteins and genetic material) and foreign elements (such as viruses or other pathogens). Cyclic GMP-AMP synthase (cGAS) is an innate immune system signaling protein that detects DNA floating in a cell. If cGAS detects a foreign presence, it opens a molecular pathway to combat the invader.
However, because HIV-1 is an RNA virus, it produces very little DNA – so little, in fact, that scientists are baffled as to how cGAS and the innate immune system detect and distinguish it from our own DNA.
Scientists at Scripps Research discovered that activating the innate immune system against HIV-1 requires a two-step security check. The first step involves an essential protein, polyglutamine binding protein 1 (PQBP1), recognizing the outer shell of the HIV-1 virus as soon as it enters the cell and before it can replicate. PQBP1 then coats and decorates the virus, acting as a call to cGAS. cGAS activates additional immune-related pathways against the virus once the viral shell begins to disassemble.
The researchers were initially surprised to discover that innate immune activation against HIV-1 requires two steps, as most other DNA-encoding viruses only activate cGAS in one step. This is similar to technologies that use two-factor authentication, such as requiring users to enter a password and then respond to a confirmation email.
This two-part mechanism also opens the door to vaccination approaches that can take advantage of the immune cascade that occurs before the virus can begin to replicate in the host cell, after PQBP1 has decorated the molecule.
“While the adaptive immune system has been the main focus for HIV research and vaccine development, our discoveries clearly show the critical role the innate immune response plays in detecting the virus,” says Sunnie Yoh, Ph.D., first author of the study and senior staff scientist in Chanda’s lab. “In modulating the narrow window in this two-step process — after PQBP1 has decorated the viral capsid, and before the virus is able to insert itself into the host genome and replicate — there is the potential to develop novel adjuvanted vaccine strategies against HIV-1.”
These findings illuminate how our bodies respond to other autoimmune or neurodegenerative inflammatory diseases by shedding light on the workings of the innate immune system. PQBP1, for example, has been shown to interact with tau, the protein dysregulated in Alzheimer’s disease, and to activate the same inflammatory cGAS pathway. The researchers will continue to look into how the innate immune system contributes to disease onset and progression, as well as how it differentiates between self and foreign cells.