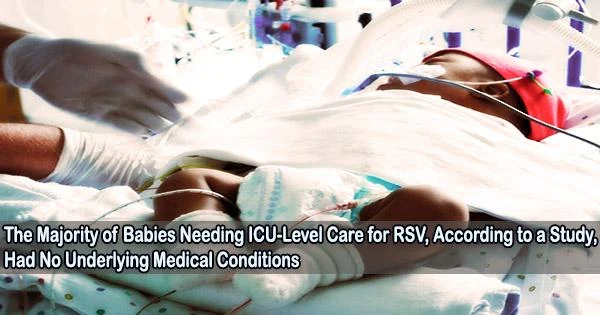
According to a recent study published in JAMA Network Open, the majority of newborns admitted to the intensive care or high acuity unit for respiratory syncytial virus (RSV) infections during fall 2022 were previously healthy and delivered at term.
The results of this study lend support to the adoption of preventative measures in all newborns to safeguard them against RSV, the most common cause of lower respiratory tract infections (LRTI) and hospitalizations globally.
According to the Centers for Disease Control and Prevention (CDC), RSV causes between 57,000 and 80,000 hospitalizations in children under the age of five, with one in five RSV-positive hospitalized children being admitted to intensive care units.
As part of the RSV Pediatric Intensive Care registry, 600 newborns from 39 hospitals in 27 states were examined for the features and prognosis of RSV-related critical illness.
The registry conducted prospective surveillance during the RSV seasonal peak in 2022. During the two-month period, the investigators found:
- The median age for infants requiring intensive care was 2.6 months
- 169 (28%) were premature
- 487 (81%) had no underlying medical conditions
- 143 (24%) received invasive mechanical ventilation
“Most of the infants in our study receiving ICU-level care were young, healthy and born at term,” said lead investigator Natasha Halasa, MD, MPH, Craig Weaver Professor of Pediatrics in the Division of Pediatric Infectious Diseases at Monroe Carell Jr. Children’s Hospital at Vanderbilt. “Although mortality was rare, our findings emphasize the significant illness caused by RSV in young infants.”
However, most infants in our study admitted to the ICU with severe RSV did not have an underlying medical condition. Therefore, our data support the need for RSV preventative interventions targeting all infants to reduce the burden of severe RSV illness, including nirsevimab, the long-acting RSV-neutralizing monoclonal antibody. The drug was recently approved by the Food and Drug Administration, and a maternal vaccine for RSV prevention is under consideration.
Natasha Halasa
According to Halasa and co-corresponding author Angela Campbell, MD, MPH, from the CDC, children with a history of prematurity or specific underlying medical conditions, such as congenital heart disease, neurologic or neurodevelopmental/neuromuscular disorders, chronic lung disease, and immunocompromising conditions, are at a higher risk for the potentially fatal RSV disease.
Palivizumab, a monoclonal antibody that prevents RSV-associated LRTI, is only approved for use in high-risk neonates.
“However, most infants in our study admitted to the ICU with severe RSV did not have an underlying medical condition. Therefore, our data support the need for RSV preventative interventions targeting all infants to reduce the burden of severe RSV illness, including nirsevimab, the long-acting RSV-neutralizing monoclonal antibody. The drug was recently approved by the Food and Drug Administration, and a maternal vaccine for RSV prevention is under consideration,” Halasa said.
“These products may protect both high-risk and healthy infants from medically attended RSV-associated LRTI.”
On August 3, 2023, the Advisory Committee on Immunization Practices (ACIP) unanimously recommended nirsevimab for all infants younger than eight months, born during or entering their first RSV season and for children ages eight to 19 months who are at increased risk of severe RSV disease and entering their second RSV season. Additionally, ACIP unanimously recommended inclusion of nirsevimab in the Vaccines for Children program.
Only two of the 17 infants who were eligible for palivizumab received the medication, according to the current study. This underlines administration challenges and emphasizes the necessity of making sure that all eligible patients receive therapeutic interventions on time. Nirsevimab just needs one shot as opposed to monthly shots, therefore it’s thought that uptake and compliance will increase.
“We hope that our study findings will aid in the design of future RSV prophylactic and maternal RSV vaccine effectiveness and usage studies and recommendations,” Halasa said.