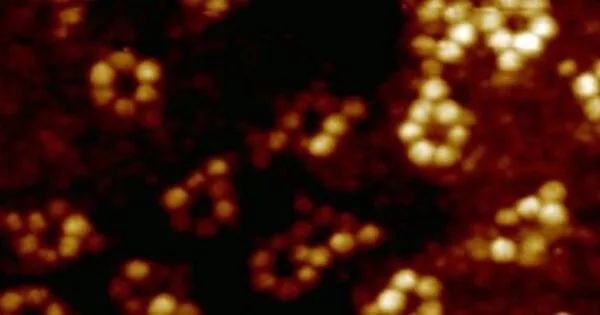
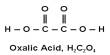
X-ray cystallography is a popular technique for determining crystal atomic structure. However, rather than imaging individual atoms directly, this technique relies on the scattering of X-rays by an ordered arrangement of atoms in a crystal lattice.
Saw Wai Hla, an Ohio University Professor of Physics and Argonne National Laboratory scientist, led a team of scientists from Ohio University, Argonne National Laboratory, the University of Illinois-Chicago, and others in taking the world’s first X-ray SIGNAL (or SIGNATURE) of just one atom. This ground-breaking achievement was supported by the US Department of Energy’s Office of Basic Energy Sciences and has the potential to change the way scientists detect materials.
X-rays have been used in a variety of applications, from medical examinations to airport security screenings, since their discovery by Wilhelm Roentgen in 1895. Curiosity, NASA’s Mars rover, is also equipped with an X-ray device to investigate the composition of the rocks on Mars. The identification of the type of materials in a sample is an important application of X-rays in science. The amount of materials in a sample required for X-ray detection has been greatly reduced over the years, thanks to the development of synchrotron X-ray sources and new instruments.
To date, the smallest amount of a sample that can be X-rayed is in an attogram, which is about 10,000 atoms or more. This is due to the X-ray signal produced by an atom being extremely weak so that conventional X-ray detectors cannot be used to detect it. According to Hla, it is a long-standing dream of scientists to X-ray just one atom, which is now being realized by the research team led by him.
Atoms can be routinely imaged with scanning probe microscopes, but without X-rays one cannot tell what they are made of. We can now detect exactly the type of a particular atom, one atom-at-a-time, and can simultaneously measure its chemical state.
Saw Wai Hla
“Atoms can be routinely imaged with scanning probe microscopes, but without X-rays, one cannot tell what they are made of. We can now detect exactly the type of a particular atom, one atom-at-a-time, and can simultaneously measure its chemical state,” explained Hla, who is also the director of the Nanoscale and Quantum Phenomena Institute at Ohio University. “Once we are able to do that, we can trace the materials down to an ultimate limit of just one atom. This will have a great impact on environmental and medical sciences and maybe even find a cure that can have a huge impact for humankind. This discovery will transform the world.”
Their paper, published in the scientific journal Nature on May 31, 2023, and gracing the cover of the print version of the scientific journal on June 1, 2023, details how Hla and several other physicists and chemists, including Ph.D. students at OHIO, used a purpose-built synchrotron X-ray instrument at the XTIP beamline of Advanced Photon Source and the Center for Nanoscale Materials at Argonne National Laboratory.
The team chose an iron atom and a terbium atom for demonstration purposes, both of which were inserted into their respective molecular hosts. To detect an X-ray signal from a single atom, the researchers used a specialized detector made of a sharp metal tip positioned in close proximity to the sample to collect X-ray excited electrons – a technique known as synchrotron X-ray scanning tunneling microscopy, or SX-STM. In SX-STM, X-ray spectroscopy is triggered by photoabsorption of core level electrons, which forms elemental fingerprints and is effective in directly identifying the elemental type of the materials.
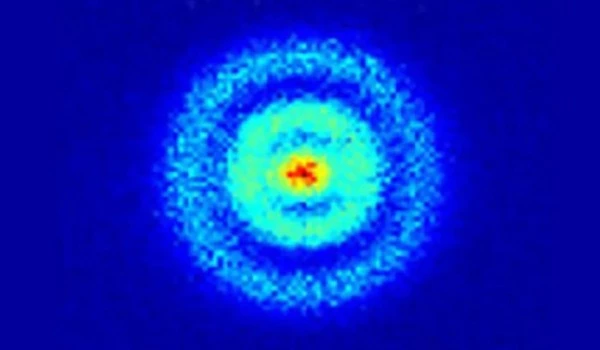
According to Hla, the spectrums are like fingerprints, each one being unique and able to detect exactly what it is.
“The technique used, and concept proven in this study, broke new ground in X-ray science and nanoscale studies,” said Tolulope Michael Ajayi, who is the first author of the paper and doing this work as part of his Ph.D. thesis. “More so, using X-rays to detect and characterize individual atoms could revolutionize research and give birth to new technologies in areas such as quantum information and the detection of trace elements in environmental and medical research, to name a few. This achievement also opens the road for advanced materials science instrumentation.”
For the last 12 years, Hla has been involved in the development of an SX-STM instrument and its measurement methods together with Volker Rose, a scientist at the Advanced Photon Source at Argonne National Laboratory.
“I have been able to successfully supervise four OHIO graduate students for their Ph.D. theses related to SX-STM method development over a 12-year period. We have come a long way to achieve the detection of a single atom X-ray signature,” Hla said.
Hla’s research focuses on nano and quantum sciences, with a particular emphasis on understanding the chemical and physical properties of materials at the most fundamental level – on an individual atom basis. The team’s main goal was to use this technique to investigate the environmental effect on a single rare-earth atom, in addition to achieving an X-ray signature of one atom.
“We have detected the chemical states of individual atoms as well,” Hla explained. “By comparing the chemical states of an iron atom and a terbium atom inside respective molecular hosts, we discover that the terbium atom, a rare-earth metal, is relatively isolated and does not change its chemical state, whereas the iron atom interacts strongly with its surroundings.”
Many rare-earth materials are used in everyday devices such as cell phones, computers, and televisions, to name a few, and play an important role in the development and advancement of technology. Scientists can now identify not only the type of element but also its chemical state, allowing them to better manipulate the atoms inside different material hosts to meet the ever-changing needs in various fields. Furthermore, they have developed a new method known as “X-ray excited resonance tunneling or X-ERT” that allows them to detect how orbitals of a single molecule orient on a material surface using synchrotron X-rays.