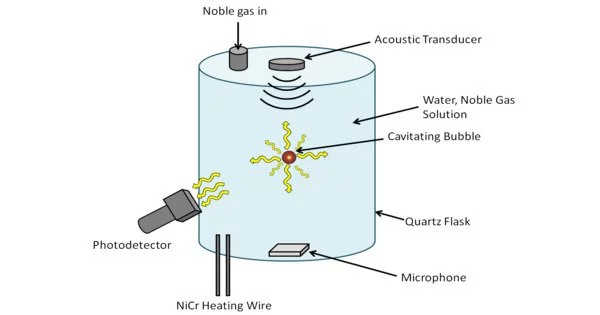
The emission of light from imploding bubbles in a liquid when stimulated by sound is known as sonoluminescence. It is a phenomenon that occurs when tiny gas bubbles suspended in a liquid are exposed to intense sound waves. The term “sonoluminescence” is derived from the Latin words “sonus” (sound) and “lumen” (light).
Sonoluminescence begins with the formation of a gas-filled bubble in a liquid, usually water. The bubble is then subjected to high-intensity sound waves, which are typically in the ultrasonic frequency range. The passage of sound waves through the liquid causes the bubble to rapidly expand and contract in size. The bubble’s cyclic compression and rarefaction results in extremely high temperatures and pressures at its center.
Sonoluminescence was discovered at the University of Cologne in 1934. It happens when a strong enough sound wave causes a gaseous cavity within a liquid to collapse quickly, releasing a burst of light. The phenomenon can be seen in both single-bubble and multi-bubble sonoluminescence (SBSL and MBSL). Peter Jarman proposed in 1960 that sonoluminescence is thermal in nature and may result from microshocks within collapsing cavities. Later experiments revealed that the temperature inside the bubble could reach 12,000 kelvins during SBSL.
The precise mechanism of sonoluminescence is unknown, but various hypotheses include hotspot, bremsstrahlung, and collision-induced radiation. Temperatures in sonoluminescent systems have been speculated to reach millions of kelvins, potentially causing thermonuclear fusion, but this idea has been met with skepticism by other researchers. The phenomenon has been observed in nature as well, with the pistol shrimp being the first known example of an animal producing light via sonoluminescence.
Under these extreme conditions, the gas inside the bubble can reach temperatures of tens of thousands of degrees Kelvin, comparable to the surface of the Sun. As a result, the gas emits light in the form of flashes or pulses. The emitted light is typically in the form of short bursts of ultraviolet (UV) radiation, but it can also include visible light and even faint flashes of X-rays.
The exact mechanism underlying sonoluminescence is still unknown and remains an active area of research. Several theories have been proposed, including the possibility of energy concentrating at the center of the bubble, the occurrence of rapid and intense chemical reactions, or even the formation of a miniature plasma state.
Scientists are interested in sonoluminescence because of its unique properties and potential applications. It’s an intriguing example of how energy can be converted from one form to another, with implications for fields like acoustics, fluid dynamics, and even nuclear fusion research. However, harnessing sonoluminescence for practical applications remains difficult, and more research is required to fully understand its underlying mechanisms and potential applications.