Cotton Fiber and its Origin:
Cotton is the oldest and most important textile fibre in the world. Historian can hardly trace cotton to its true origin. The best we know is that the English word “Cotton” is derived from Arabic word katan (or qutn, kuteen). Each cotton fibre is a unicellular hair collected from the seed of the cotton plant. Cotton plant belongs to the order Malvales, the family Malvaceae, the tribe Gossypieae and the genus Gossypium. It grows in subtropical climates. Most of the cotton cultivated in Asia, Africa, America Egypt, India and other places. Cotton had grown in more than 80 countries in worldwide.
Cotton fibre is the purest source of cellulose and the most significant natural fibre. The economic significant of cotton in global market is evident by its majority share (50%) among fibres for apparel and textiles goods . Cotton is a soft, fluffy staple fibre that grows in a boll or protective capsule, around the seeds of cotton plants of the genus Gossypium. Nearly 90% of cotton fibres are cellulose.
Classification of Cotton Fibre:
Commercial cotton may be classified broadly into following classes with reference of the staple length.
- Short staple: Length: 3/8 inch to 1 inch. Includes the coarse, low grade fibers which have often low strength and little luster, the fibers are generally of 13-22 microns diameter and are of 1.5 -2.9 dtex. Many Indian and Asiatic cottons come into this category.
- Medium staple: Length: 0.5 inch to 1.25 inch. Includes the medium strength, medium luster cottons which from bulk of the world crop. The fibers are generally 12-17 microns diameter and are of 1.4-2.2 dtex., American upland and some Peruvian types come into this category cotton.
- Long staple: Length: 1inch to 2.5 inch. Includes the fine, luster fibers which from the top quality cottons. The fibers are generally 10-15 microns diameter and 1.1-1.8 dtex. Sea Island, Egyptian and American pima cottons are this category. This high quality cottons are often the most difficult to grow and are in comparatively short supply.
Chemical Composition of Cotton:
Cotton fibers are composed of mostly cellulose. The specific chemical compositions of cotton fibers vary by their varieties, growing conditions (soil, water, temperature, pest etc.) and maturity, Due to this reasons it was expressed the average value of different component of cotton. Therefore chemical composition of cotton is given below .
Table Chemical composition of cotton fibre.
Cellulose | 94% |
Protein | 1.3% |
Pectin | 1.2% |
Ash | 1.5% |
Fat/wax | 0.6% |
Sugar | 0.8% |
Others | 0.9% |
Cellulose:
Cellulose is the principle constituent of all plant life. The main features of the chemical structure are well known and have been summarized many times. The elemental analysis shows that cellulose contains 44.4% carbon, 6.2% hydrogen and 49.4% of oxygen. It is a liner polymer of anhydroglucose units linked in 1 and 4 positions by ß-glucoside links. The empirical formula of cellulose (C6H10O5) corresponds to a polyanhydride of glucose. The two terminal glucose residues of a cellulose molecule contain two different end groups. One contains a reducing hemiacetal group in the position C1 and known as the reducing end group where as the other contains an extra secondary hydroxyl group in the position C4 and its known as the non reducing end group.
Properties of Cotton Fibre:
Cotton, a unique cellulosic fibre, possesses an outstanding range of properties making it one of the most popular apparel fibres worldwide.
Physical Properties:
a. Length and width:
Cotton is the shortest natural fibre used in commercially in the textile industry ranging from 0.5 – 2.5 inches in length. The width of typical cotton fiber may vary between 12 to 20 microns.
b. Shape:
A typical, matured raw cotton fibre looks like a flattened tube and the cross section is oval or kidney shape. Under microscope, it is a long twisted ribbon.
c. Strength:
Cotton is a moderately strong fibre. Tenacity of a cotton fiber is 26.5-44 cN/Tex and the extension at break is about 8-10%
d. Elasticity:
Cotton is relatively rigid fibre (Less elastic). At 2% extension is has an elastic recovery of 74%, at 5% extension, the elastic recovery is only 45%.
e. Elongation:
Cotton does not stretch easily. It has an elongation at break of 5-10%.
f. Fineness:
10-17 microns (1.4-2.2 dtex) for good quality fibre, 15-17 microns (1.1-1.8dtex) for medium quality fibre, 13-22 microns (1.5-2.5dtex) for low quality fibre.
g. Porosity:
Cotton fibre is somewhat porous, and consequently, it absorbs moisture readily.
h. Moisture regains:
Cotton has a moisture regain of 8.5%. At 100% humidity, cotton has an absorbency of 25-27%.
i. Color:
Normally, the color of cotton is creamy-white.
j. Handle:
Cotton is naturally very soft and comfortable, that is why it is particularly favored for garments that get close to the skin.
k. Luster:
Cotton fibres have a natural luster which is due to the natural polish on the surface and its nearly circular cross-sectional shape. The smooth, hard primary coat of cellulose contains waxes which no doubts contribute to the luster of the fiber.
L. Specific Gravity:
Cotton fibre specific gravity is 1.54
Chemical Properties of Cotton:
Effect of Acid:
Cotton fibres are not affected by cold week acids. But whenever it is brought into contact with hot diluted acid or cold concentrated acids, degradation of cellulose takes place and the fibres loss their strength. Cotton fibre can fully dissolved in high concentrated mineral acid.
Effect of Alkalis:
Cotton has an excellent resistance to alkalis. It swells in caustic alkali (mercerization) but not damaged. But the fibre is affected by hot alkali particularly in the presence of air. In this case, NaOH promotes cellulose oxidation by atmospheric oxygen resulting in the formation of oxycellulose. For this reason, it is suggested that to carry out the scouring of cotton in a hooded machine.
Effect of Organic Solvent:
There are few solvents that will dissolve cotton completely. It has a high resistance to normal solvents but it dispersed by the copper complexes cuprammonium hydroxide and cupriethylene diamine, and by concentrated (70%) H2SO4.
Effect of Oxidizing Agent:
For chemical treatment, cotton fibre is treated with different types of oxidizing agents like sodium hypochlorite, sodium perborate, hydrogen peroxide, sodium chlorite etc. These reagents lead to chemical attack initially in the functional groups and then progressively cause the chain scission, lowering the DP and reducing the tensile strength.
Action of Micro Organism:
Cotton fibres can resist moths and most insects, but it can be attacked by fungi and bacteria. Mildew, for example, causes weakening and rotting tine cotton fiber often characterized by a musty smell. The presence of starchy material as a sizing or a finishing agent also promotes the growth of the mildew. Mildews and bacteria will flourish on cotton under moist and hot condition.
Action of Water:
Water, which is very strong polar in nature, easily attracted by the polar –OH groups of the cotton fiber. So that water is able to penetrate into the cellulose network of the cotton fiber. However, its porous structure also allows ready penetration of water molecules between the fibrils and into the amorphous region of the polymer, where they can easily form hydrogen bond with free –OH group of cellulose. This water absorption causes swelling of the fibre.
Due to swelling, cotton fibre shows greater strength in the wet state than dry. This is because of the screw-shaped arrangement of the fibrils in cotton fibre, which are supposed to be pressed more firmly against each other by the swelling action.
Other Properties of Cotton:
Effect of Heat:
Cotton fibres show an excellent resistance to thermal decomposition. They have the ability to conduct heat energy and do not allow any destructive heat accumulation. Thus they can withstand high temperature, but the stability depends to a great extend on the heating time and temperature. Heating generally causes dehydration and decomposition of cotton fibre. Prolonged heating at 100°C shows the cotton no visible change. Heating at 120°C for several hours shows the cotton little or no change in strength, but begins to turn yellow gradually. Cotton decomposes markedly at 150°C. At 240°C intensive decomposition takes place with the formation of liquid and gaseous products of different composition. At 400-500°C all gaseous decomposition products disappeared and hard residue (carbon) remains.
Effect of Age:
Cotton shows only small loss strength when stored carefully. It can be kept in the warehouse for long time without showing any significant deterioration. After fifty years of storage cotton may differ only slightly from fibre a year or two old. Ancient samples of cotton fabric taken from toms more than 500 years old had four-fifth of strength of new material.
Effect of Sun Light:
There is a gradual loss of strength when cotton is exposed to sunlight for a long time and the fibre turns yellow. The degradation of cotton by oxidation when heated is promoted and encouraged by sunlight. It is particularly severe at high temperature and in the presence of moisture. Much of the damage is caused by ultra-violet light and by the shorter waves of visible light. Under certain conditions the effects of weathering in direct sunlight can be serious. The cotton can be protected to some degree by using suitable dyes.
Silk Fiber and its Origin:
Silk is a natural protein fibre, some forms of which can be woven into textiles. A variety of silks, produced by caterpillars and mulberry silkworm, have been known and used in China, South Asia, and Europe since ancient times. The strands of raw silk as they are unwound from the cocoon consist of the two silk filaments mixed with sericin and other materials. About 75 % of the strand is silk i.e. fibroin and 23 % is sericin; the remaining materials consist of fat and wax (1.5 %) and mineral salts (0.5 %). As a natural protein fibre silk has a significant attraction towards natural dyes.
Silk are formed by the polymerization of amino acids (with the general formula NH2·CHR·COOH) by means of peptide links (—CO·NH—) to give long-chain molecules with the general formula-
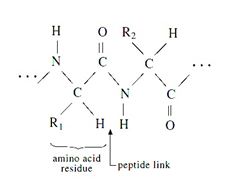
Figure : Silk fibre structure
(R1, R2 represents the side groups present in the silk structure.)
Physical properties of silk: Silk fibers from the Bombyx mori silkworm have a triangular cross section with rounded corners, 5-10 μm wide. The fibroin-heavy chain is composed mostly of beta-sheets, due to a 59-mer amino acid repeat sequence with some variations. The flat surfaces of the fibrils reflect light at many angles, giving silk a natural shine. The cross-section from other silkworms can vary in shape and diameter: crescent-like for Anaphe and elongated wedge for tussah. Silkworm fibers are naturally extruded from two silkworm glands as a pair of primary filaments (brin), which are stuck together, with sericin proteins that act like glue, to form a bave. Bave diameters for tussah silk can reach 65 μm. See cited reference for cross-sectional SEM photographs.
Silk has a smooth, soft texture that is not slippery, unlike many synthetic fibers.
Silk is one of the strongest natural fibers but loses up to 20% of its strength when wet. It has a good moisture regain of 11%. Its elasticity is moderate to poor: if elongated even a small amount, it remains stretched. It can be weakened if exposed to too much sunlight. It may also be attacked by insects, especially if left dirty.
One example of the durable nature of silk over other fabrics is demonstrated by the recovery in 1840 of silk garments from a wreck of 1782: ‘The most durable article found has been silk; for besides pieces of cloaks and lace, a pair of black satin breeches, and a large satin waistcoat with flaps, were got up, of which the silk was perfect, but the lining entirely gone … from the thread giving way … No articles of dress of wollen cloth have yet been found.
Silk is a poor conductor of electricity and thus susceptible to static cling.
Unwashed silk chiffon may shrink up to 8% due to a relaxation of the fiber macrostructure, so silk should either be washed prior to garment construction, or dry cleaned. Dry cleaning may still shrink the chiffon up to 4%. Occasionally, this shrinkage can be reversed by a gentle steaming with a press cloth. There is almost no gradual shrinkage nor shrinkage due to molecular-level deformation.
Natural and synthetic silk is known to manifest piezoelectric properties in proteins, probably due to its molecular structure.
Silkworm silk was used as the standard for the denier, a measurement of linear density in fibers. Silkworm silk therefore has a linear density of approximately 1 den, or 1.1 dtex.
Chemical properties of silk:
Silk emitted by the silkworm consists of two main proteins, sericin and fibroin, fibroin being the structural center of the silk, and serecin being the sticky material surrounding it. Fibroin is made up of the amino acids Gly-Ser-Gly-Ala-Gly-Ala and forms beta pleated sheets. Hydrogen bonds form between chains, and side chains form above and below the plane of the hydrogen bond network.
The high proportion (50%) of glycine, which is a small amino acid, allows tight packing and the fibers are strong and resistant to breaking. The tensile strength is due to the many interceded hydrogen bonds, and when stretched the force is applied to these numerous bonds and they do not break.
Silk is resistant to most mineral acids, except for sulfuric acid, which dissolves it. It is yellowed by perspiration.
Fabric Fastness Properties:
One of the most important properties is that the stability of color of a dyeing or its fastness. It is the important requirement requirements of valuable customers. During use a dyed material is exposed to a variety of agencies that can cause its color fade i.e. the color decay from deeper to paler shade. These changes occur because of decomposition of the dye molecules in the fibre or because of their removal into the external medium. The colored textiles show different resistance power to different agencies such as light, wash, rubbing, perspiration, water, bleach, acid, alkali etc. The aspect of these agencies many colorfastness tests are using to determine the stability of color in textile. Dyed and printed materials are affected (color fade/bleed) by some agencies such as washing, light, water, dry cleaning, chlorine, perspiration and ironing. Color fastness is the resistance of the color to fade or bleed by these agencies. Fastness tests are described in detail in documents from professional organization such as the Society of dyers and Colourists (SDC),the American Association of Textile Chemists and Colorists (AATCC) and American Society for Testing and Materials(ASTM). Most European countries adopt the fastness standards of the International Organization for standardization (ISO) as their national standards. Color fastness is usually assessed separately with respect to;
- Change in the color of the specimen being tested that is color fading.
- Staining of undyed material which is in contact with the specimen during the test that is bleeding of color.
In order to give numerical assessment of each of these effects two sets of standard grey scales are used, one for color change another for color staining.