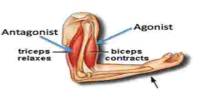
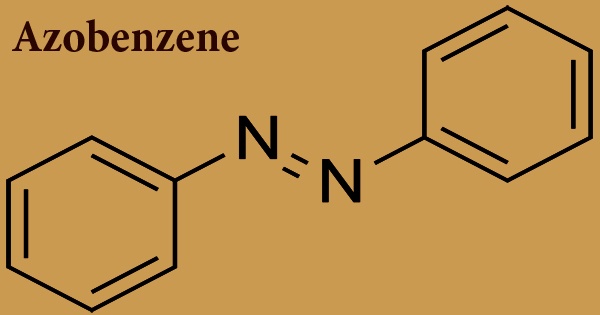
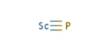Good manufacturing practice (GMP) is a production and testing practice that helps to ensure a quality product. Many countries have legislated that pharmaceutical and medical device companies must follow GMP procedures, and have created their own GMP guidelines. Good Manufacturing Practice is required for minimize human error; prevent the contamination of pharmaceuticals and deterioration of quality and ensure high quality of product.
Good Manufacturing Practice