Introduction
The eye is the organ of choice for the practical implantation or injection of a drug delivery system. The intraocular structures are easily (and visually) accessible and, at the same time, it is confined and isolated from the circulation by the inner and outer blood-retinal barriers. These barriers allow for a real local delivery of active products. The eye’s anatomy includes two cavities (the anterior chamber and vitreous space) in which ocular fluids circulate and allow for a wide space of implantation. Furthermore, the surface of tissues to be targeted is large but their volume small. Finally, the eye benefits from an “immune privilege” particularly observed in the anterior chamber and in the sub retinal space, limiting the risk for exaggerated inflammatory reaction to foreign antigens and cell graft rejection. However, due to the vulnerability of its visual functions, drug targeting of the eye by simple means is inefficient. Therefore, it remains an organ for which successful drug delivery may be the limiting factor for a successful therapeutic strategy. The challenge of future therapeutic strategies in ophthalmology is the ability to integrate in clinical practice optimized and safe drug delivery implants. Ocular implantable drug delivery systems are to be specifically designed and adapted to the targeted tissue or cell type, the physico-chemical properties of the active compound to be used and to the desired kinetic of intraocular release. Non-biodegradable implants offered in the early 70s the advantages of long-lasting release and reduced host response. Nowadays, new eroding polymers ensuring sustained release and limited induced-inflammation have been designed. The erosion rate and spontaneous degradation of these polymers can be modulated to allow for the desired intraocular kinetics of drug release to take place. Biodegradable polymers can be used to form solid or injectable implants or they can be used to encapsulate particular systems as nano and microparticles. Particulate systems can be injected through thin needles and have different behavior and distribution in the ocular media associated with their size and composition. Polymers can be devised as viscous or semi-solid materialswhich can be localized within the eye and used as a slow release intraocular “implant” after a simple injection. Finally, thermo or light sensitive polymers can be used and appropriately delivered as needed by localized application of heat or various light lengths.
Ocular Implants for Drug Delivery
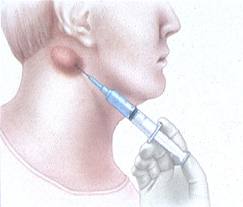
Advances in materials science, biomedical engineering, and surgical techniques have encouraged the development of a variety of intraocular therapeutic devices. One class of intraocular devices includes implants for drug delivery, designed to improve the ability to deliver therapeutic drug levels into the eye. Most sight-threatening diseases occur in the back of the eye and frequently involve the retinal tissue. Local drug delivery with implants can overcome the many barriers that inhibit effective treatment of retinal diseases with traditional mechanisms of drug delivery, such as with eye drops or medications given systemically (i.e., by oral or intravenous route). The modified mucosal surface that covers the cornea is a significant barrier to topically applied eye drop medications (Fig. 2-1). The tight junctions between the basal epithelial cells of the cornea and the tear fluid drainage causes less than 5% of topically applied drug to permeate the cornea and reach intraocular tissues. As a result, drugs delivered with eye drop formulations are generally limited to treating tissues in the front of the eye. Examples include topical antimicrobials used to treat bacterial conjunctivitis or ocular antihypertensive drops that function by reducing the aqueous humor production at the level of the ciliary body (located in the front of the eye) to treat glaucoma. An attempt to deliver drugs to the posterior structures of the eye with systemic medications is impeded by the blood-retinal-barrier, which consists of the tight junctional complexes of the retinal pigment epithelium and retinal capillaries.This barrier reduces the penetration of most drugs given systemically to the posterior segment of the eye and hinders the treatment of many retinal diseases. Although high doses of systemically administered medications may be able to penetrate the blood-retinal-barrier and provide therapeutic drug levels to the back of the eye, serious systemic side effects may occur. Delivery of drugs directly into the eye by intravitreal injection through the pars plana is another approach to achieve therapeutic levels of drug to treat retinal diseases (Fig. 2-2). However, given the short half life of most drugs injected into the vitreous, frequent injections 1-3 times per week are required to maintain therapeutic drug levels. As a result, intravitreal drug injections are very useful in treating acute bacterial infections of the eye (i.e., endophthalmitis)8 that may require one or two injections, but are not well tolerated by patients that may require months or years of injections to treat chronic eye diseases such as AMD or cytomegalovirus (CMV) retinitis (Fig. 2-3). Furthermore, frequent injections increase the risk of retinal detachment, vitreous hemorrhage, endophthalmitis, and cataracts.
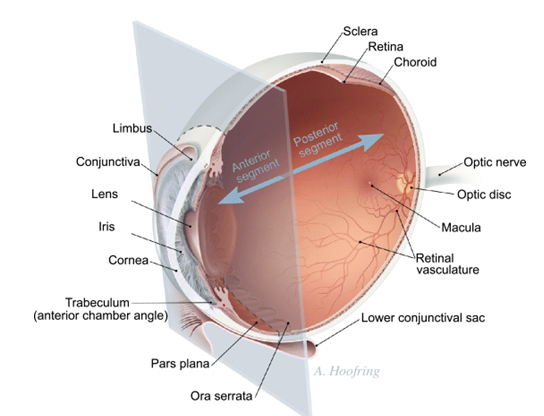
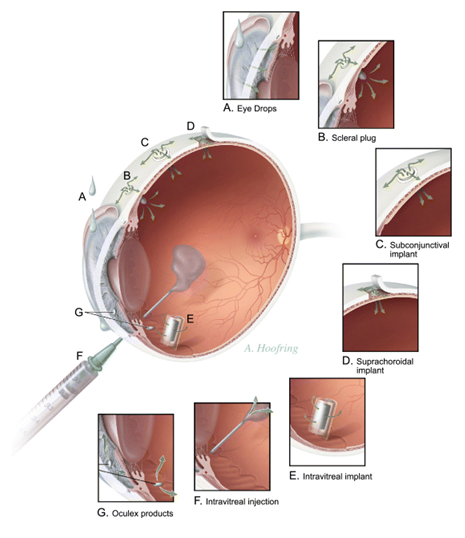
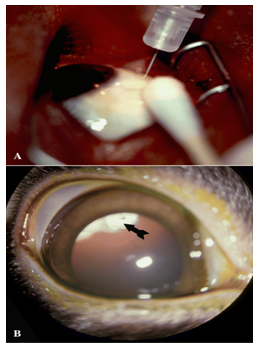
Figure 2- 3.(A) External photograph of the eye showing injection of an antiviral medication into the vitreous cavity to treat CMV retinitis in a patient with advanced HIV-infection. These injections are repeated twice per week. (B) External photograph of rabbit eye showing front of the eye with pupil widely dilated. A corticosteroid medication (arrow) had been injected a week before and positioned in the vitreous cavity behind the lens.
Intraocular implants for drug delivery are capable of bypassing the barriers to intraocular drug absorption and avoid frequent procedures required using intravitreal injection therapy. Drug release rates of implants can be controlled so that therapeutic drug levels are maintained in the eye, while avoiding toxic or subtherapeutic levels. Moreover, higher intraocular drug levels can be achieved using implants compared with systemic or topical administration, making the drug more effective for treating a variety of retinal diseases.
Polymeric Systems for Ocular Implants
A polymer, natural or synthetic is a substance that is combined with a drug or other active agent to release drug in a predesigned manner. The basic objective of controlled drug release is to achieve more effective therapies by eliminating the potential for both under- and overdosing. Other advantages are the maintenance of drug concentration within a desired range, fewer administrations, optimal drug use and increased patient compliance.
Among anatomical locations, eye is one of the important sites that has been treated and studied for the optimum drug delivery by controlled drug delivery devices. Conventional dosage forms such as solutions, suspensions, gels and ointments are used but face incompetence due to rapid drug drainage from site of application and different physiological factors of the eye. Polymeric inserts and discs have been developed to overcome such difficulties. Inserts allow for accurate dosing, reduced systemic absorption and better patient compliance resulting from reduced frequency of administration and lower incidence of systemic side effects. Moreover, inserts are least affected by nasolacrimal drainage and tear flow thus provide reliable drug release and longer residence.
Pre-requisite for the Polymers as an Ingredient of Ocular Formulation
Polymers were originally intended for non-biological uses and selected because of their desirable physical properties . Other desired properties of a material to be used as a polymer are chemical inertness, absence of leachable impurities, appropriate physical structure with minimal undesired aging and readily processable material. Polymeric devices need to be biocompatible, inert, non-irritant to ocular tissues, mechanically strong, comfortable to the patient, capable of achieving high drug loading, safe from accidental release, simple to administer and remove and easy to fabricate and sterilize.
Drug Delivery Modules by Polymeric Devices
There are three primary mechanisms by which active agents can be released from a delivery system: diffusion, degradation and swelling followed by diffusion. Any or all of these mechanisms may occur in a given release system. Diffusion occurs when a drug or other active agent passes through the polymer that forms the controlled-release device. The diffusion can occur on a macroscopic scale (through pores in the polymer matrix) or on a molecular level (by passing between polymer chains). When a polymer and an active agent are mixed to form a homogeneous system, it is referred to as a matrix system. Diffusion occurs when the drug passes from the polymer matrix into the external environment. As the release continues, its rate decreases with this type of system, since the active agent has a progressively longer distance to travel and therefore requires a longer diffusion time to release. For the reservoir systems, the drug delivery rate can remain fairly constant. In this design, a reservoir—whether solid drug, dilute solution, or highly concentrated drug solution within a polymer matrix is surrounded by a film or membrane of a rate-controlling material. It is the structure of the polymer layer surrounding the reservoir that effectively limits the release of the drug. Since, this polymer coating is essentially uniform and of a nonchanging thickness, the diffusion rate of the active agent can be kept fairly stable throughout the lifetime of the delivery system.
Recently some drug delivery systems have been developed in which only one side of the device deliver the drug. All of the previously described systems are based on polymers that do not change their chemical structure beyond what occurs during swelling. However, a great deal of attention and research effort has been concentrated on biodegradable polymers. These materials degrade within the body as a result of natural biological processes, eliminating the need of removal from drug delivery system after release of the active agent. Most biodegradable polymers are designed to degrade as a result of hydrolysis of the polymer chains into biologically acceptable and progressively smaller compounds. In some cases like polylactides, polyglycolides and their copolymers, the polymers will eventually break down to lactic acid and glycolic acid, enter the Kreb’s cycle, and further broken down into carbon dioxide and water which is excreted through normal processes. Degradation may take place through bulk hydrolysis, in which the polymer degrades in a fairly uniform manner throughout the matrix. The present work comprehends the various polymer systems studied for drug delivery through ocular inserts and their relevant outcomes for improving poor ocular bioavailability.
Description of the polymers used for intraocular drug delivery implants
Non-biodegradable solid implants
Polyvinyl alcohol (PVA)–ethylene vinyl acetate (EVA)
PVA is a permeable polymer acting as the framework of the implant and regulating the rate of release. EVA and silicon are permeable to certain lipophilic substances but, due to their hydrophobic character, they are relatively impermeable to hydrophilic drugs and can therefore limit the surface area for the release of this type of drugs.EVAismainly used as a membrane in reservoir systems. These polymers mechanism of action is based on diffusion of a fluid (water) into the device dissolving the drug pellet, creating a saturated solution released to themediumby diffusion out of the device.As long as the inside solution is saturated with drug, the release rate is constant. The non-biodegradable polymer devices do not produce an initial burst of drug. Very long-lasting (more than a year) and controlled release has been achieved using this type of implants, with higher concentrations measured in the vitreous than in the aqueous humor and very low serum concentrations. The major drawbacks for the use of this type of device are the need for a surgical implantation and the need to remove it after it empties. Complications associated with the implantation of these devices have been observed and include: vitreous hemorrhage, retinal detachment, endophtalmitis, cystoid macular edema and the formation of tenacious epiretinal membranes. Development of intrascleral or subconjunctival implantation can reduce complications linked with these devices.
PCF (polysulfone capillary fiber)
Polysulfone is a water impermeable polymer permeable to lipophilic as well as hydrophilic compounds. Advantage of this polymer is the presence of deep macrovoids in the outer membrane which increase the surface area for drug diffusion and release. Bioactivity of the encapsulated drug is preserved as the fabrication process does not require chemical reaction, heat or solvent exposure. PCF implants can be sterilized but they have to be removed once emptied. Kinetics studies of carboxyfluorescein dye release from PCF showed in the rabbit eye constant intravitreal level for up to 45 days without any evident signs of ocular toxicity.
Biodegradable solid implants
Poly lactic acid (PLA), poly glycolic acid (PGA), and poly lactic-co-glycolic acid (PLGA)
Polymers and copolymers of PLA and PLGA are synthesized by condensation reaction at high temperature. Once implanted, bulk erosion occurs causing a burst of encapsulated drug. This phenomenon takes place following the cleavage of polymeric chains by enzymatic and non-enzymatic hydrolysis. The half life of pure PLA is longer than that of the copolymers while PLGA (50:50) has the shortest half life in tissue. Drug release from these devices usually follows a three phases pattern: initial drug-burst, diffusive phase (regulated by the speed of polymer degradation, the surface area of the device, the percentage of loaded and its water solubility) and a final drug burst. The latest phenomenon is generally uncontrollable, poorly predictable and therefore, largely undesirable. The choice of polymers must thus be based on the drug characteristics and the foreseen duration of its release. Many attempts to improve the release pattern of these polymeric implants have been made (reviewed in). Minimizing the final burst, achieving a pseudo-zero order kinetic of drug release and control of its speed was achieved by using 2 PLA polymers of different molecular weight and at different ratios. These devices can be modulated into various shapes including rods, plugs, pellets, discs and sheets. Accordingly, they can be implanted into the anterior chamber, the vitreous cavity through the pars plana, or into the intrascleral space.
Polycaprolactones (PCL)
PCL is synthesized at high temperature by an open ring polymerization of themonomer å-caprolactone. It is semi-crystalline and hydrophobic compared to PLA. PCL degradation by cleavage of the ester bound produces small polymeric fragments which diffuse from the matrix and undergo phagocytosis. Drug release with PCL porous reservoir can be obtained for more than 250 days with a zero-kinetic order. Copolymerizations of caprolactone leading to poly-glycolide-colactide- co-caprolactone (PLGC) have been developed and studied in corneal allograft and uveitis.
Polyanhydrides
Polyanhydrides can be synthesized by melt-polycondensation, dehydrochlorination and dehydrative coupling[16]. The most studied polyanhydride is the 1,3-bis(carboxyphenoxypropane) (PCPP) with sebacic acid (SA), a more hydrophilic polymer. Pure PCPP has an extremely long lifetime (over 3 years), whereas after copolymerization with 80% SA it is reduced to a few days. The rate of hydrolysis can be controlled by modulating the ratio of the two polymers. Increasing sebacic acid leads to a less hydrophobic polymer and thus, to a faster rate of hydrolysis of the polymeric chains. These polymers are degraded by surface erosion and have a very good biocompatibility.
Viscous and injectable polymers:
poly ortho esters (POE) POE are hydrophobic polymers degraded by surface erosion confined to the polymer–water interface and follow a zero-order kinetics when placed in a biological environment. This type of drug release is controlled by gradual surface degradation of the polymer and drug release rather than drug diffusion. Since the 1970s, four families of POE were synthesized. POE I and POE II families were not used for in vivo ophthalmologic studies and will not be described in detail here. POE III and IV have interesting characteristics valuable for ophthalmic therapeutic potential applications. Therefore we describe these polymers here.
Third generation of POE is, at room temperature, in a gel-like conformation. This state of the polymer allows the incorporation of therapeutic agents by simple mixing without the need for solvents. Moreover these viscous POE can be injected directly into the eye with an appropriate needle. POE III are prepared by transesterification between trimethyl orthoacetate and 1,2,6-hexanetriol. Since POE contain pH sensitive links in the polymeric backbone, degradation rate of the polymer can be controlled by incorporating acidic substances into the polymer matrix to increase the erosion rate, or on the contrary basic ones to stabilize the polymer backbone. Moreover, addition of a basic compound improved POE tolerance by neutralizing the acidic microenvironment formed during POE degradation. The molecular weight of the polymer also strongly influences its drug release kinetic profile; in vitro, 35 kD and 33 kD polymers showed a drug release ability for 1 and 7 days respectively. Classic gamma irradiation sterilization induces POE III polymer degradation. Therefore, aseptic preparation of the polymer is recommended. Storage has to be carried out in sealed glass bottles under argon atmosphere.
Biocompatibility of POE III to ocular tissues has been extensively investigated. Subconjunctival implants releasing antimetabolites in the rabbit eye led to an acute inflammation which resolved rapidly.
Different types of Ocular Implants
Ocular implants have many advantages over more traditional methods of drug administration to the eye, including delivering constant therapeutic levels of drug directly to the site of action and bypassing the blood–brain barrier. Release rates are typically well below toxic levels, and higher concentrations of the drug are therefore achieved without systemic side effects. In general, subconjunctival implantation is used for anterior-segment diseases, whereas intravitreal and suprachoroidal methods are typically used to treat posterior-segment diseases. Intrascleral implants can be used for either. A significant amount of crossover can occur, and a drug may be delivered to both segments, regardless of placement.
Subconjunctival implants are inserted through a small incision in the conjunctiva and placed in contact with the sclera. Intrascleral devices, implanted in a small scleral pocket at one-half the total scleral thickness, place the drug closer to its site of action than conventional transcleral devices, making it more useful for treatment of posterior-segment diseases with less systemic absorption of the drug than subconjunctival or peribulbar injections.
Intravitreal placement of ocular implants has the advantage of delivering a drug directly to target tissues of the posterior segment. The implant is inserted into the vitreous through a sclerotomy site or injected with an applicator, which has the advantage of no sutures with needle delivery. The site of implantation is commonly over the pars plana, which is anterior to the insertion of the retina and posterior to the lens, and this is the area least likely to damage those structures.
The structures of polymeric devices for controlled, sustained release are classified as nonbiodegradable and biodegradable (Figure 5-1). Nonbiodegradable implants have the advantage of steady, controlled release of a drug during potentially long periods of time (years) and the disadvantage of removal and/or replacement when the drug is depleted. Biodegradable implants have the advantage of being able to be fashioned into many shapes, they are amenable for injection as an office procedure, they do not require removal, and they increase the half-life of the drug.
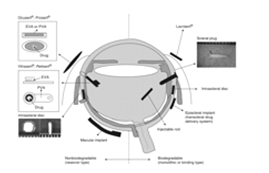 Nonbiodegradable
Nonbiodegradable
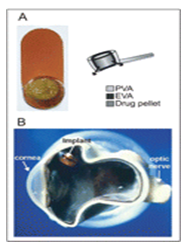
Figure 5-2: The Vitrasert implant. (a) Front view showing drug pellet containing Ganciclovir on the left and the schematic on the right, with the drug pellet surrounded by two polymers, PVA and EVA. (b) A cadaver eye from a patient with HIV infections showing a cross-sectional view of the location of the Vitrasert implant fixed at the pars plana. EVA = ethylene vinyl acetate; PVA = polyvinyl alcohol. Reproduced by permission from Davis et al. (2004). Curr Opin Mol Therap 6, 195–205. © 2004 Thomson Corporation.
Nonbiodegradable corticosteroid implants of dexamethasone and fluocinolone have been investigated for their potential to treat inflammatory diseases of the eye and diseases that involve fibrovascular proliferation.

Figure 5-3:Cyclosporine intravitreal sustained-release implant and the Retisert implant. (a) Double-pellet (left) and single-pellet (right) cyclosporine implants to deliver at different release rates. (b) Photograph of human eye with double implant in vitreous cavity. Retisert (fluocinolone acetonide) implant showing (c) front and (d) side views. Reproduced by permission from Davis et al. (2004). Curr Opin Mol Therap 6, 195–205. © 2004 Thomson Corporation.
Cyclosporine has been investigated for its use as an immuno-suppressant in cases of chronic uveitis in humans and horses and keratoconjunctivitis sicca in dogs with an intravitreal or episcleral (suprachoroidal space) implant (Figure 5-3b) with a silicone-based matrix design that is effective for at least 6 months.
Delivery of large-molecular-weight compounds has been relatively unsuccessful when incorporated into reservoir implants. One exception is encapsulated cell technology (ECT), which is a cell-based delivery system that can be used to deliver therapeutic agents to the eye. Genetically modified cells are packaged in a hollow tube of semipermeable membrane, which prevents immune-cell entry and allows nutrients and therapeutic molecules to diffuse freely across the membrane. Two ends of the polymer section are sealed, and a titanium loop is placed on the anchoring end, which is implanted at the pars plana and anchored to the sclera. Currently, ciliary neurotrophic factor (CNTF, NT-501), which has been shown to protect the retina from degeneration in many animal models, safely completed phase 1 clinical trials as delivered by an ETC implant containing NTC-201 cells, which are genetically engineered human retinal pigment epithelium cells that overexpress CNTF.
Biodegradable Matrix Implants
Matrix implants are typically used to treat acute-onset diseases that require a loading dose followed by tapering doses of the drug during a 1-day to 6-month time period . They are most commonly made from the copolymers poly-lactic-acid (PLA) and/or poly-lactic-glycolic acid (PLGA), which degrade to water and carbon dioxide (Figure 5-4). The rate and extent of drug release from the implant can be decreased by altering the relative concentrations of lactide (slow) and glycolide (fast), altering the polymer weight ratios, adding additional coats of polymer, or using hydrophobic, insoluble drugs. The release of drug generally follows first-order kinetics with an initial burst of drug release followed by a rapid decline in drug levels. The advantage over a nonbiodegradable implant is that biodegradable implants do not require removal, as they dissolve over time . Biodegradable implants also allow flexibility in dose and treatment from short duration (weeks) to longer duration (months to a year), depending on the polymer PLA/PLGA ratio, which is another benefit in tailoring drug delivery to disease progression, because dose and treatment requirements may change over 
Figure 5-4:Posterior-segment drug-delivery system (PS DDS) composed of PLA/PLGA demonstrating a rod for delivery of dexamethasone intravitreally. PLA = poly-lactic acid; PLGA = poly-lactic-glycolic acid. Reproduced by permission of Kupperman et al. (2007) Arch Ophthalmol 125, 309–17. © 2007 American Medical Association.
Effective sustained delivery has been achieved using a variety of drugs: antiviral, antifungal, antimetabolic, immunosuppressive agents, and steroids. Biodegradable ocular inserts placed in the lower conjunctival sac and mixed with nonbiodegradable polymers have been used with atropine, pilocarpine, and gentamicin.
Examples of ocular implants in the treatment of some ocular disease:
Sustained release intravitreal ganciclovir implant
Sustained release local drug delivery has had a profound impact on the treatment of cytomegalovirus retinitis. Before the use of highly active antiretroviral therapy (HAART), treatment of cytomegalovirus required lifelong systemic medications. Unfortunately, systemic administration of ganciclovir, foscarnet, or cidofovir is associated with significant side effects. Ganciclovir and foscarnet can cause myelosuppression and renal toxicity respectively.Because of concern over the toxicity of systemic administration of anti-CMV therapy, ophthalmologists began injecting drugs directly into the vitreous.. However, the relatively short half life of ganciclovir in the eye required one to two weekly injections to maintain therapeutic drug levels. The need for multiple injections is not only inconvenient for patients, but also increases the risk of ocular complications.
The development of a sustained release ganciclovir implant has allowed local drug delivery without the need for multiple injections. The ganciclovir implant is produced by partially coating a compressed pellet of ganciclovir with ethylene vinyl acetate, a compound that is impermeable to the drug, and a surrounding coat of polyvinyl alcohol. This implant releases drug at a rate of 1 μg/h and leads to intravitreal drug levels that are much higher than those achieved with maintenance intravenous therapy.Current implants deliver drug for approximately 8 months. The implant is surgically placed through the pars plana, and has been well tolerated in the eye.
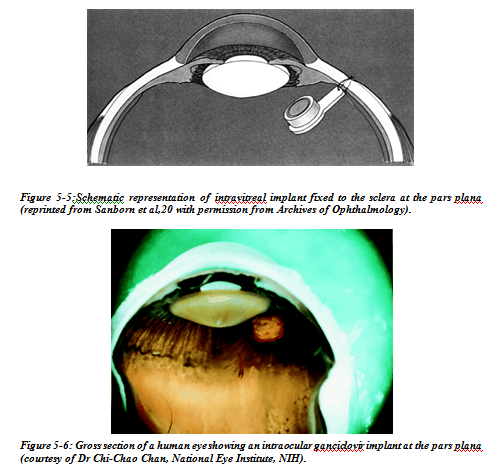
Sustained release intravitreal cyclosporin implant
Cyclosporin is an effective secondary agent in the treatment of uveitis. Usually reserved for patients with advanced bilateral disease despite high doses of prednisone, its main effect is on the recruitment and activation of T cells. It is believed to act by interfering with interleukin 2 (IL-2) in the activation of T cell genes. Although CD4 lymphocytes are the main target, CD8 cells are also suppressed. Systemic administration is usually through the oral route as a suspension. The gastrointestinal absorption is variable, however, with a range of 4–60%. Some of the most common side effects at a dose of 10 mg/kg include paraesthesias and hyperaesthesia (40%), hypertension (24%), epigastric burning (20%), hypertrichorism and gingivitis (20%). Hypertension, renal toxicity, and an increase in the predisposition to malignancy such as lymphoma are perhaps the most threatening. Many patients require long term management, increasing the risk of complications and making careful monitoring of their renal function, blood pressure, and surveillance for malignancy an important part of their management. In addition, baseline abnormalities in renal function tests often preclude the use of cyclosporin in certain patients.
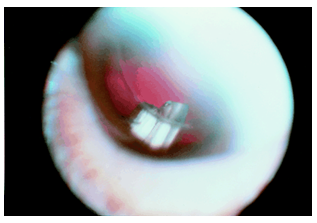
Figure 5-7:Slit lamp photograph showing a cyclosporin implant in the inferotemporal quadrant. The patient is pseudophakic.
Sustained release intravitreal corticosteroid implants
Corticosteroids play an important part in the current management of ocular inflammatory disease. Topical, periocular, or systemic administrations remain the mainstay of treatment. Corticosteroids also may have an important role in the management of ocular diseases that involve neovascularisation, wound healing, and fibrosis, such as proliferative vitreoretinopathy and subretinal neovascularisation.
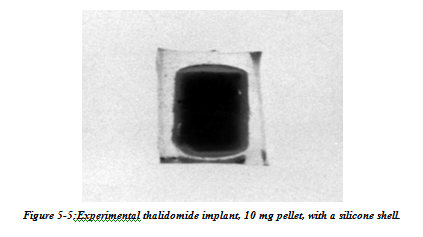
Intraocular delivery of corticosteroids could play in important part in the treatment of ocular diseases which involve inflammation, fibrous proliferation, and neovascularisation by sustaining intravitreal steroid levels which could otherwise not be achieved. A dexamethasone implant has been shown, for example, to effectively inhibit the development of experimental uveitis. Sustained release technology allows the local delivery of a number of drugs for the treatment of ocular disease. Compounds that are potentially effective yet restricted by their systemic side effects can now be reconsidered as treatments. Angiostatic agents, for example, are currently under investigation for the treatment of age related macular degeneration. Despite the potential benefits, systemic administration of these agents can have significant side effects. Thalidomide, for example, is known to be a powerful angiostatic agent. Its systemic side effects, however, include peripheral neuropathy, central nervous system depression, and embryotoxicity, and have limited the dosages administered to patients for the treatment of subretinal neovascularisation. Systemic inhibition of angiogenesis in older patients can also interfere with the development of collateral circulation, which has a role in the prevention of central nervous system as well as cardiac ischaemic events. Implant that successfully releases therapeutic intraocular doses of thalidomide, and could be used for the treatment of subretinal neovascularisation have been developed(Fig 5-5).
Implants have already been developed for other applications. An intravitreal implant, for example, has been developed for the sustained release of calcium channel blockers for the potential treatment of glaucoma. Calcium channel antagonists may improve optic nerve head circulation as well as improve visual field testing in patients with normal tension glaucoma. An implant that can deliver calcium channel antagonists directly to the eye could avoid systemic haemodynamic effects such as hypotension.
An intravitreal implant of trimetrexate has also been developed for the treatment of intraocular lymphoma. Like its parent drug, methotrexate, trimetrexate inhibits the enzyme dihydrofolate reductase, thereby interfering with the production of reduced folate cofactors. Certain cellular reactions responsible for the production of nucleotides are dependent on these cofactors.Trimetrexate, therefore, interferes with DNA and RNA synthesis (S phase) of the cell, making it an effective agent against rapidly dividing lymphoma cells. Systemic administration, however, can result in haematological, hepatic, renal, and gastrointestinal toxicity requiring the concomitant administration of leucovorin. In addition, the systemic clearance of trimetrexate can be very rapid, with a terminal serum half life of less than 12 hours. Frequent systemic administrations at high doses would be required for the treatment of intraocular disease, and the development of an intraocular implant could clearly be of benefit in these patients.
Other novel designs such as the use of nanospheres or subconjunctival matrices will provide more flexibility in the application of this technology, allowing the mode of drug delivery to be customised to the particular properties of a compound and the disease being treated. Nanospheres and other vehicles made of biodegradable materials will allow sustained release drug formulations to be injected into the eye. Their short life spans make them ideal for use in diseases such as infectious endophthalmitis, retinopathy of prematurity, and post-traumatic vitreous proliferation. Lipophilic prodrugs can also be formulated as liposomal preparations, and can act as natural microsomes and prolong intravitreal concentrations. Such preparations could be injected intravitreally, and achieve therapeutic concentrations for short though significant periods of time. Recently, Cheng and colleagues published encouraging data on such a formulation of a lipid prodrug of foscarnet for the treatment of CMV retinitis.Subconjunctival matrices could be the delivery of choice for highly lipophilic agents which more easily penetrate the eye and do not require intraocular administration to achieve therapeutic levels.
Finally, gene therapy may be a unique technology allowing long term delivery of therapeutic agents. Viral vectors can be inserted into the eye for the transfection of genes to specific tissues. Genes coding for specific proteins can be incorporated into cells, leading to the long term expression of proteins or enzymes which are deficient or absent in a particular disease, or to the normalisation of gene expression in diseases where overproduction leads to pathology. Such genetic manipulation would allow effective treatment of ocular disease with minimal surgical intervention. However, this technology has a number of drawbacks in its current development. Disease mechanisms must be clearly established and the involved genes identified before this approach is applicable. Viral vectors can be used to target specific ocular tissues and have been shown to deliver genetic material effectively, but are limited by the immune response targeted against the virus, their transduction efficiency, the size of the DNA they can deliver, and the length of gene expression. Non-viral vectors may be safer and less costly, but are limited by their poor transfection rates and expression efficiency. Current research in this field, however, is making this technology a promising and plausible treatment modality for ocular disease.
Challenges and obstacles of ocular pharmacokinetics and drug delivery
In clinical practice the anterior segment of the eye (cornea, conjunctiva, sclera, anterior uvea) can be treated with topical ocular eye drops, the most commonly used dosage form in ocular drug treatment. Unfortunately the eye drops are rapidly drained from the ocular surface and, therefore, the time for drug absorption is only a few minutes and bioavailability is very low, typically less than 5%. Bioavailability and duration of activity may be increased modestly byprolonged action dosage forms, but they have not gained wide acceptance by the patients. Even from the modified formulations the ocular drug absorption is limited by the corneal and conjunctival epithelial barriers of the eye. Topical ocular medications do not reach the posterior segment drug targets. Posterior segment (retina, vitreous, choroid) can be treated by high drug doses given intravenously or by intravitreal administration. Currently there is rapidly growing interest in the posterior segment drug delivery. This is caused by the advances in the understanding of the pathophysiological processes in the retina and choroid. Many posterior segment diseases cannot be treated effectively with current methods. These diseases include age related macular degeneration, retinitis pigmentosa, diabetic retinopathies, and neural changes induced by glaucoma. Posterior segment delivery of both small molecules and larger bio-organic compounds, such as proteins and DNA, is problematic. Only drugs with wide therapeutic index (such as antibiotics) can be given in massive doses to blood stream to treat the posterior segment. Intravitreal injection, on the other hand, is invasivemethod andmay cause even endophthalmitis. Therefore, it is not considered to be an ideal method of drug administration to large numbers of patients. Therefore, there is increasing interest to develop new prolonged action dosage forms for subconjunctival and periocular administration. Another option is efficient drug targeting from the blood stream to the retinal pigment epithelium or choroidal vasculature. Breakthroughs in such approaches should enable more patient friendly, safer and efficient treatment of posterior segment diseases with antibodies, oligonucleotides, genes, and growth factors. Even though the main emphasis in current ocular drug delivery research is focused on posterior eye segment, there are still challenges remaining. For example the treatment of lacrimal gland and the disorders of lacrimal secretion are still poorly understood fields. Comprehensive reviews about drug delivery and pharmacokinetics of the eye have been published earlier . Therefore, this review provides only an overview of the main aspects of ocular pharmacokinetics.
Ocular pharmacokinetics
The main routes of drug administration and elimination from the eye have been shown schematically in the following figure:
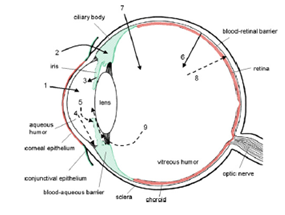 Figure 6-1:In the above figure, schematic presentation of the ocular structure with the routes of drug kinetics illustrated. The numbers refer to following processes: 1) transcorneal permeation from the lacrimal fluid into the anterior chamber, 2) non-corneal drug permeation across the conjunctiva and sclera into the anterior uvea, 3) drug distribution from the blood stream via blood-aqueous barrier into the anterior chamber, 4) elimination of drug from the anterior chamber by the aqueous humor turnover to the trabecular meshwork and Sclemm’s canal, 5) drug elimination from the aqueous humor into the systemic circulation across the blood-aqueous barrier, 6) drug distribution from the blood into the posterior eye across the blood-retina barrier, 7) intravitreal drug administration, 8) drug elimination from the vitreous via posterior route across the blood-retina barrier, and 9) drug elimination from the vitreous via anterior route to the posterior chamber.
Figure 6-1:In the above figure, schematic presentation of the ocular structure with the routes of drug kinetics illustrated. The numbers refer to following processes: 1) transcorneal permeation from the lacrimal fluid into the anterior chamber, 2) non-corneal drug permeation across the conjunctiva and sclera into the anterior uvea, 3) drug distribution from the blood stream via blood-aqueous barrier into the anterior chamber, 4) elimination of drug from the anterior chamber by the aqueous humor turnover to the trabecular meshwork and Sclemm’s canal, 5) drug elimination from the aqueous humor into the systemic circulation across the blood-aqueous barrier, 6) drug distribution from the blood into the posterior eye across the blood-retina barrier, 7) intravitreal drug administration, 8) drug elimination from the vitreous via posterior route across the blood-retina barrier, and 9) drug elimination from the vitreous via anterior route to the posterior chamber.
The barriers
Drug loss from the ocular surface After instillation, the flow of lacrimal fluid removes instilled compounds from the surface of the eye. Even though the lacrimal turnover rate is only about 1 ìl/min the excess volume of the instilled fluid is flown to the Another source of non-productive drug removal is its systemic absorption instead of ocular absorption. Systemic absorption may take place either directly from the conjunctival sac via local blood capillaries or after the solution flow to the nasal cavity. Anyway, most of small molecular weight drug dose is absorbed into systemic circulation rapidly in fewminutes. This contrasts the low ocular bioavailability of less than 5%. Drug absorption into the systemic circulation decreases the drug concentration in lacrimal fluid extensively. Therefore, constant drug release from solid delivery system to the tear fluid may lead only to ocular bioavailability of about 10%, since most of the drug is cleared by the local systemic absorption anyway.
Lacrimal fluid-eye barriers
Corneal epithelium limits drug absorption from the lacrimal fluid into the eye. The corneal barrier is formed upon maturation of the epithelial cells. They migrate from the limbal region towards the center of the cornea and to the apical surface. The most apical corneal epithelial cells form tight junctions that limit the paracellular drug permeation. Therefore, lipophilic drugs have typically at least an order of magnitude higher permeability in the cornea than the hydrophilic drugs. Despite the tightness of the corneal epithelial layer, transcorneal permeation is themain route of drug entrance from the lacrimal fluid to the aqueous humor. In general, the conjunctiva is more leaky epithelium than the cornea and its surface area is also nearly 20 times greater than that of the cornea. Drug absorption across the bulbar conjunctiva has gained increasing attention recently, since conjunctiva is also fairly permeable to the hydrophilic and large molecules.
Blood-ocular barriers
The eye is protected fromthe xenobiotics in the blood stream by blood-ocular barriers. These barriers have two parts: blood-aqueous barrier and blood-retina barrier. The anterior blood-eye barrier is composed of the endothelial cells in the uvea. This barrier prevents the access of plasma albumin into the aqueous humor, and limits also the access of hydrophilic drugs from plasma into the aqueous humor. Inflammation may disrupt the integrity of this barrier causing the unlimited drug distribution to the anterior chamber. In fact, the permeability of this barrier is poorly characterised. The posterior barrier between blood stream and eye is comprised of retinal pigment epithelium (RPE) and the tight walls of retinal capillaries. Unlike retinal capillaries the vasculature of the choroid has extensive blood flow and leaky walls. Drugs easily gain access to the choroidal extravascular space, but thereafter distribution into the retina is limited by the RPE and retinal endothelia. Despite its high blood flow the choroidal blood flow constitutes only a minor fraction of the entire blood flow in the body. Therefore, without specific targeting systems only a minute fraction of the intravenous or oral drug dose gains access to the retina and choroid. Unlike blood brain barrier, the blood-eye barriers have not been characterised in terms of drug transporter and metabolic enzyme expression. From the pharmacokinetic perspective plenty of basic research is needed before the nature of blood-eye barriers is understood.
Routes of ocular drug delivery
There are several possible routes of drug delivery into the ocular tissues . The selection of the route of administration depends primarily on the target tissue. Traditionally topical ocular and subconjunctival administrations are used for anterior targets and intravitreal administration for posterior targets. Design of the dosage form can have big influence on the resulting drug concentrations and on the duration of drug action.
Topical ocular
Typically topical ocular drug administration is accomplished by eye drops, but they have only a short contact time on the eye surface. The contact, and thereby duration of drug action, can be prolonged by formulation design (e.g. gels, gelifying formulations, ointments, and inserts). During the short contact of drug on the corneal surface it partitions to the epithelium and in the case of lipophilic compounds it remains in the epitheliumand is slowly released to the corneal stroma and further to the anterior chamber. After eye drop administration the peak concentration in the anterior chamber is reached after 20–30 min, but this concentration is typically two orders of magnitude lower than the instilled concentration even for lipophilic compounds. From the aqueous humor the drug has an easy access to the iris and ciliary body, where the drug may bind to melanin. Melanin bound drug may form a reservoir that is released gradually to the surrounding cells, thereby prolonging the drug activity. Distribution to the lens is
much slower than the distribution to the uvea. Unlike porous uvea, the lens is tightly packed protein rich structure where drug partitioning takes place slowly. Drug is eliminated from the aqueous humor by two main mechanisms: by aqueous turnover through the chamber angle and Sclemm’s canal and by the venous blood flow of the anterior uvea. The first mechanism has a rate of about 3 µl/min and this convective flow is independent of the drug. Elimination by the uveal blood flow, on the other hand, depends on the drug’s ability to penetrate across the endothelial walls of the vessels. For this reason, clearance from the anterior chamber is faster for lipophilic than for hydrophilic drugs. Clearance of lipophilic drugs can be in the range of 20–30 ìl/min. In those cases, most of drug elimination takes place via uveal blood flow. Halflifes of drugs in the anterior chamber are typically short, about an hour. The volumes of distribution are difficult to determine due to the slow equilibration of drug in the ocular tissues. The estimates in rabbits range from the volume of aqueous humor (250 ìl) up to 2 ml. In the latter case, the slow drug distribution to the vitreous is included in the volume of distribution. This distribution is slow, because the lens prohibits drug access to the vitreous. Flow of aqueous humor from the posterior chamber to the anterior chamber is another limiting factor. Some part of topically administered drugs may absorb across the bulbar conjunctiva to the sclera and further to the uvea and posterior segment (Fig. 6-1). This is an inefficient process, but may be improved by dosage forms that release drug constantly to the conjunctival surface. The role of this non-corneal route of absorption depends on the drug properties. Generally more hydrophilic and larger molecules may absorb via this route. They have particularly poor penetration across the cornea, and therefore, the relative contribution of the non-corneal is more eminent. Delivery across the conjunctiva and further to the posterior segment would be desirable, but unfortunately the penetration is clinically insignificant.
Subconjunctival administration
Traditionally subconjunctival injections have been used to deliver drugs at increased levels to the uvea. Currently this mode of drug delivery has gained new momentum for various reasons. The progress in materials sciences and pharmaceutical formulation have provided new exciting possibilities to develop controlled release formulations to deliver drugs to the posterior segment and to guide the healing process after surgery (e.g. glaucoma surgery). Secondly, the development of new therapies for macular degeneration (antibodies, oligonucleotides) must be delivered to the retina and choroid. After subconjunctival injection drug must penetrate across sclera which is more permeable than the cornea. Interestingly the scleral permeability is not dependent on drug lipophilicity. In this respect it clearly differs fromthe cornea and conjunctiva. Even more interesting is the surprisingly high permeability of sclera to the large molecules of even protein size. Thus, it would seem feasible to deliver drugs across sclera to the choroid. However, delivery to the retina is more complicated, because in this case the drug must pass across the choroid and RPE. The role of blood flow is well characterized kinetically but the based on the existing information, there are good reasons to believe that drugs may be cleared significantly to the blood streamin the choroid.Pitkänen et al. showedrecently thatRPEis tighter barrier that sclera for the permeation of hydrophilic compounds . In thecase of small lipophilic drugs they have similar permeabilities. More complete understanding of the kinetics in sclera, choroid and RPE should help to develop medications with optimal activity in the selected posterior target tissues. Combination of the kinetic knowledge and cell selective targeting moieties offer very interesting possibilities.
Intravitreal administration
Direct drug administration into the vitreous offers distinct advantage of more straightforward access to the vitreous and retina (Fig. 6-1). It should be noted, however, that delivery from the vitreous to the choroid is more complicated due to the hindrance by the RPE barrier. Small molecules are able to diffuse rapidly in the vitreous but the mobility of largemolecules, particularly positively charged, is restricted. Likewise, the mobility of the nanoparticles is highly dependent on the structure. In addition to the diffusive movement convection also plays a role. The convection results from the eye movements. After intravitreal injection the drug is eliminated by two main routes: anterior and posterior. All compounds are able to use the anterior route. This means drug diffusion across the vitreous to the posterior chamber and, thereafter, elimination via aqueous turnover and uveal blood flow. Posterior elimination takes place by permeation across the posterior bloodeye barrier. This requires adequate passive permeability (i.e. small molecular size, lipophilicity) or active transport across these barriers. For these reasons, large molecular weight and water-solubility tend to prolong the half-life in the vitreous. Drugs can be administered to the vitreous also in controlled release formulations (liposomes, microspheres, implants) to prolong the drug activity.
Therapeutic applications of ocular implants
Implantation in the anterior segment of the eye
Sub-conjunctival implantation
Sub-conjunctival implantation at the site of a filtering surgery .The main therapeutic application in the anterior segment of the eye has been the slow delivery of agents to control certain stages of the wound healing response for the prevention of glaucoma filtering surgery failure. Non-biodegradable PVA–EVA copolymers were studied in 1989 in vitro. The therapeutic compound 5-FU was incorporated into Elvax discs of 4 mm diameter, and was liberated for 2 weeks. Subconjunctival implantation of these devices containing 12 mg 5-FU lead to 1 mg/day liberation of 5-FU for 10 days in the rabbit eye; in the same study, reduced intraocular pressure was observed for more than 3 months in the monkey, although 5-FU was released for 2 weeks only. These implants were also used subconjunctivally in four patients undergoing high-risk trabeculectomy with a mean follow-up of 2.5 years. In three of the four studied patients, intraocular pressure remained low with stabilization of the visual field and without any unwarranted events. A mild inflammatory reaction was observed in the rabbit and the guinea pig eyes 1 week after implantation persisting for at least 6 weeks. These implanted discs disintegrated within 4 weeks after implantation. Devising small PLGA cylinders, more constant release rates were achieved. In the rabbit eye, a release rate of 1.20 ìg/h for more than 18 days was recorded. Total degradation of the implant was achieved around 86 days after implantation. No infection or inflammatory reactions were detected during the study period. A zero-order release was obtained using implants with a central hole within their coat. In these cases, therapeutic drug concentrations in the conjunctiva and in the sclera were observed up to 8 days post implantation.
Intracameral implantation to prevent corneal graft rejection or post-operative inflammation
Intracorneal implants were used in experimental pre clinical studies for the evaluation of anti-angiogenic and/ or anti inflammatory properties of pharmacological agents. Studies using Elvax-40 pellets demonstrated the potential role of prostaglandins, growth factors and chemoattractants in the cascade of events leading to corneal neovascularization. The role of various cytokines and interleukins was also studied and unveiled the potent angiogenic effect of interleukin-1 sequestered in Elvax-40 pellets used as slow release intracorneal implants. Similar intracorneal implants demonstrated the potential anti-angiogenic properties of TGF-â. Most of the clinical therapeutic evaluations were carried out with biodegradable Surodex® (Oculex® Pharmaceutical, Inc. Sunnyvale, CA, USA) PLGA devices. Insertion of 0.3 mg cyclosporine A (CsA) implant in the anterior chamber (AC) of rat eyes was found to be more efficient than subconjunctival delivery. Insertion of a 1×2 mm implant loaded with 0.5 mg of CsA in the anterior chamber of the rabbit eye led to CsA detection in all layers of the cornea for 3 months. Constant low levels of CsA were detected in the aqueous humor but none was observed in the serum. A mild fibrin reaction around the pellet occurred near the insertion site and persisted for approximately 1 to 2 weeks after surgery. This reaction was associated with mild peripheral iris vascular engorgement at the site of pellet placement noted up to 2 months after the surgery in both the CsA and placebo treated eyes[59]. This vascular engorgement was not associated with cornea edema, increased IOP or increase of fibrin in the AC. The rate of implant degradation differed significantly between CsA and control pellets. The CsA implants had a slower degradation rate and remained within the AC longer than the placebo implants. The CsA implants showed surface changes only 6 weeks after insertion in theACand a size reduction of 10% to 20% at 3 months. The control pellets surface changes were observed as early as one postoperative day after insertion within the AC with 40% to 60%of its size by the 1 to 2 months post surgery. Intra cameral delivery of dexamethasone (60 ìg) loaded within the Surodex® DDS reduced markedly the corneal graft rejection processes in rat eyes. A first randomized comparative human clinical study was performed using two Surodex® implants placed either in the sulcus (35 eyes) or in the anterior chamber (36 eyes) as compared to 0.1 dexamethasone eye drops (4 times/day) after phacoemulsification and intraocular lens implantation. Laser flaremeter evaluation showed a significant reduction of the post operative inflammatory reaction in the Surodex® treated groups at day 4 and 15 post implantation without significant differences in corneal endothelial cell counts up to 1 year post operatively. No differences in efficacy or complications were found between the sulcus or the anterior chamber implantations[60,61]. In another study, the Surodex® implant (0.5 mm in diameter and 1.0 mm in length and cylindrical) containing 0.06 mg of microdispersed dexamethasone did not demonstrate any significant benefits when compared to treatment with 0.1% dexamethasone drops. Surodex® implant remnants persisted for a mean of 22.0+/-2.5 (SD) months postoperatively without causing any endothelial cell count changes.
The main advantages of intra-cameral implants compared to the use of eye drops are: a reduced systemic drug effect, a more controlled drug delivery and reduced complications related to patient compliance with frequent eye drop instillations. Intra-cameral implantation of dexamethasone releasing implants may therefore be considered in high-risk patientswith lowcompliance. The efficacy of PLGC loaded with 0.5 mg FK506 was compared to CsA loaded PLGC after implantation into the anterior chamber of 12 high rejection risks corneal grafts in rabbit eyes. The mean graft survival for the FK506-PGLC was more than 180 days with sustained drug release for 168 days.
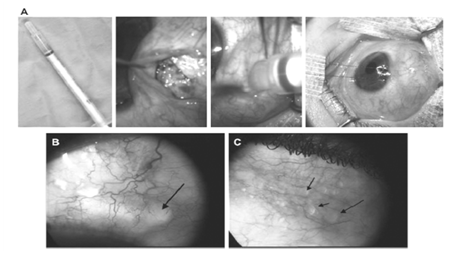
Figure 7-1: Pre-operative and post-operative pictures of POEIV/5-FU used in adjunction to filtering surgery in a patient with high risk o failureglaucoma. A: Injection of 100 µl of POEIV 5-FU at the site of deep sclerectomy, in the sub-conjunctival space, at the end of the surgery before suturing the conjunctival flap. B: Presence of the polymer is seen as a transparent material under the conjunctiva at 8 days after surgery. C: Three months after the surgery, microbubbles of POE are still observed under the conjunctiva. Note the vary good tolerance of the polymer under the conjunctiva.
Intracameral implantation for the treatment of uveitis
Surodex® (PLGA) loaded with 60 ìg dexamethasone were implanted in the anterior chamber of rat eyes after induction of uveitis (acute EIU model and chronic EAU model). Both acute and chronic inflammatory reactions were markedly inhibited in the eyes receiving the Surodex® implants. More recently, a glycolide-co-lactide-co-caprolactone copolymer (PGLC) implant loaded with 2mg CsA was evaluated. In rabbit eyes with experimental uveitis, therapeutic CsA levels were measured in the aqueous humor up to 14 weeks post-implantation and efficient anti-inflammatory effects were observed in the CsAPGCL treated eyes. Reduced systemic toxicity was noted with the CsA-PGCL treated rabbits compared to those receiving systemic CsA treatment. From a clinical point of view, intracameral implantation in an inflamed eye remains problematic. However, this strategy has practical clinical potential after complicated intra ocular surgery and in eyes with chronic uveitis refractory to conventional treatment.
Drug releasing implants in the anterior segment to prevent posterior capsule opacification (PCO)
PLA or PLGA drug delivery implants have been tested for the potential inhibition of the proliferation of lens epithelial cells and formation of secondary cataract. PLGA disks implanted in the capsular bag and containing 7 mg indomethacin allowed drug release for 3 weeks with a significant reduction in inflammation observed post surgery, but without any PCO reduction. EDTA loaded PLGA implants inhibited the migration of the secondary lens fibers to the posterior capsule and its opacification. The potential effect of an open-loop hydrogel intracapsular ring releasing 5-FU (0.25 ìg/h of 5-FU) was tested in rabbit eyes after lens phacoemulsification. No beneficial effect on the formation of PCO associated with the release of 5-FU was observed in the treated eyes. Recently, Xie et al. tested the benefits of direct subconjunctival injection of heparin to its encapsulation within posterior chamber implant in rabbit eyes and observed higher therapeutic effects in eyes receiving the intraocular implants.
Implantation in the posterior segment of the eye
Intravitreal implantation for the prevention of proliferative vitroretinopathy (PVR)
PCF intravitreal implants loaded with daunomycin in tristearin, in the rabbit eye showed dose-dependent release of the drug for 21 days. This treatment prevented tractional retinal detachment in an experimental model of PVR and was well tolerated. Dexamethasone loaded PVA–EVA implants allowed a 1.5 µg/h drug release for more than 3 months in the rabbit vitreous. Intravitreal sustained-release of triamcinolone acetonide and 5-fluorouracil (TA/5-FU) co-drug led to a reduced incidence of tractional retinal detachment in the treated eyes without any drug-related toxic effects. A cylindrical solid PLGA implant loaded with 1 mg fluorouracil and placed in the vitreous cavity of rabbits allowed drug release for almost 3 weeks and reduced the incidence of tractional retinal detachment. No toxic or mechanical effects were observed. However, as the device is free in the vitreous cavity it can cause trauma to the retina. Therefore, scleral plugs fixed at the pars plana were developed. When these plugs were loaded with 1% doxorubicin, drug release at therapeutic levels were observed in the vitreous of rabbit eyes during 1 month with reduction of the incidence of tractional retinal detachment. In 1998, Zhou et al developed a multidrug PLGA implant made of three cylindrical segments each containing one drug: 5- fluoroudine (5-Furd), triamcinolone and recombinant tissue plasminogen activator (rtPa). This device can easily be inserted through a syringe needle in the vitreous. In vitro kinetic studies showed release of 5- Furd and triamcinolone at 1 µg/day for over 4 weeks and at 10–190 µg/day for 2 more weeks. An active rate of rtPa release was detected after a lag-time of 2 days and during a shorter period of 2 weeks. However, this drug releasing implant has not been, as yet, evaluated in vivo. Two types of PLGA scleral implants (PLGA 65/35 and PLGA 50/50) were combined for the release of cis-4-hydroxyproline (CHP). PLGA 65/35 and PLGA 50/50 released the drug for 4 and 7 weeks, respectively, according to 3-phase release profile. Both implants led to synergistic therapeutic effects.
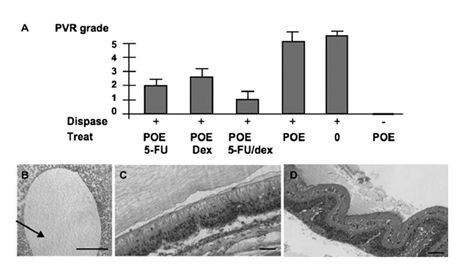
Figure 7-2: Evaluation of POE IV (POE70LA30, Mw=6900) containing 5-FU and /or dexamethasone in a rabbit model of PVE. A:Clinical score of PVR in the different groups of treatment at 30 days post treatment. B:Histological evaluation, showing the presence of the POE bubble floating in the vitreous cavity, and persisting at 30 days post injection. Note the absence of encapsulation of the POE. C: Grade 1 PVR eye, treated with POE containing both 1% dexamethasone. D: Grade 5 PVR eye, untreated with tractional total retinal detachment and fixed folds.
Polycaprolactone (PCL) has been used to deliver intravitreal 5-FU[75]. This device allowed slow release of the drug and led to a 100% protection against tractional retinal detachment. Mild vitreous hemorrhage was observed in a few of the treated eyes but no other significant complications were detected. We have evaluated the potential of intravitreous POE IV (POE70LA30, Mw=6900) implants releasing either 5-FU alone, 5-FU combined with dexamethasone or dexamethasone alone for the prevention of PVR in a rabbit model. PVR was induced in albino abbits by intravitreous injection of 0.05 UI of dispase (100 µl)[76] and graded clinically as described by Fastenberg et al. (Grade 0: No PVR, grade 1: epiretinal membranes, grade 2: focal traction, vessels abnormalities and tortuosity, grade 3: localized retinal detachment, grade 4: extended retinal detachment and peripapillar detachment, grade 5: total retinal detachment, fixed folds and retinal tears). Five groups of rabbits (n=4) were observed after dispase injection. Group I was not treated, group II received 100 µl of POE alone, group III received 100 µl of POE+1% 5FU (w/w), group IV received POE+1% Dexa (w/w), group V received 100 µl POE+1% 5-FU– 1% Dexa. Group VI received 100 µl of naked POE without dispase injection. In all groups, POE-IV was injected in the vitreous cavity of the rabbit as previously described, and dispasewas injected at the same time. In the end of the experiments, the rabbits were sacrificed and the eyes were enucleated, embedded in paraffin, sectioned, stained with hematoxylin-eosin and examined. As shown in Fig. 7-2A, POE alone did not induce any PVR, and POE alone did not affect the development of PVRin dispase-injected eyes inwhich PVR scores 4 and 5 were observed. On the other hand, PVR grades 2 to 3 were observed in eyes treated with POE containing either 1% 5–FU or 1% dexamethasone. Eyes treated with POE releasing both drugs had the lowest PVR grade (1±0.5), demonstrating that the combination of the 5-FUand dexamethasone is superior to the release of one of the drug alone. Histology at day 30 (Fig. 7-2B) shows the presence of the POE bubble still floating in the vitreous cavity. While flat retina with limited epi- retinal membrane could be observed in POE1% 5-FU– 1% dexa treated eyes (Fig. 7-2C), tractional detachments with PVR membrane were observed in control POE injected groups (Fig. 7-2D). POE IV being well tolerated, it could be used to release drugs in the vitreous cavity.
Intravitreal implantation to treat CMV retinitis
CMV retinitis is the main clinical application for PVA-EVA polymer implants. They have been developed in 1992 for the release of ganciclovir, and led to long-term(more than 80 days) drug delivery in the rabbit eye with no toxic effects. Studies in experimental animals and in humans were conducted and led to the device approval for clinical use by the Food and Drug Administration in 1996 (Vitrasert®). Concomitantly, scleral plugs fixed to the pars plana were developed byHashizoe et al. These devices were loaded with dauxorubicin hydrochloride or 25% ganciclovir. In the rabbit eye, these implants released these drugs for more than 4 weeks and 12 weeks respectively without any observed ocular toxicity. Advantages of the Vitrasert® approach are: convenience, reduced cost and lack of systemic toxicity. This strategy, however, has also potential disadvantages including endophthalmitis and increased rate of retinal detachments. It allows also for the development of nonocular cytomegalovirus disease and cytomegalovirus retinopathy in fellow, uninvolved, eyes. Vitrasert® device showed a longmedian time of CMVretinitis control. However, postoperative complications occurred in 12% of the procedures and were associated with decreased visual acuity. Thus, recommendations of the International AIDS society for Vitrasert® use in combination with anti retroviral therapy were made. Sustained release of ganciclovir was also obtained with 10% loaded PLA/PLGA (75/25) scleral implants for more than 3 months in the retina and more than 5 months in the choroid of rabbit eyes. Long-term release for 12 weeks was also obtained with PLGA scleral plugs containing 25% ganciclovir with no significant toxicity. Scleral implants made of two different PLA sizes allowed for constant drug release of ganciclovir during 6 months without a significant burst release in the late phase. Advantages of these biodegradable implants are the minimal sclerectomy required for implantation, minimizing surgical complication risk. An interesting study was conducted with 8.4% phosphorothioate oligodeoxynucleotide loaded PLGA implant which led, in vitro, to initial 20% release of the ODN followed by a pseudo-zero kinetic order of release for more than 20 days. To date, scleral plugs have not been applied clinically due to the potential induction of ocular hypotony and wound healing delay caused during the polymer biodegradation.
Intravitreal and intrascleral implants for the treatment or prevention of endophthalmitis
PLA or PLGA intraocular implants loaded with ciprofloxacin were used for the treatment of experimental endophthalmitis in rabbit eyes. This antibiotic was released in the vitreous at therapeutic levels up to 4 weeks after implantation[94]. Scleral implants loaded with fluconazole released the antifungal drug during 3 weeks. The implants were reabsorbed 4 months after implantation[95] . Rod-shaped intravitreal implants containing dexamethasone delivered the drug in rabbit normal eye for 4 weeks. Drug release however was 2.5 times more rapid in eyes after vitrectomy [96].
Intravitreal implantation to treat uveitis
Studies were conducted with PVA-EVA devices sequestering 5 mg dexamethasone and implanted in the rabbit eyes. These implants reduced significantly ocular inflammation as well as complications of experimental uveitis for 3.5 months. Concentration of 500 ng/ml cyclosporin A was also obtained for more than 6 months by intravitreal implantation of PVA– EVA devices in rabbit and monkey eyes. The very slow CsA release rate provided sustained intravitreal drug levels for up to 9 years. However, lens opacities were observed in the implanted rabbit eyes. In rabbit eyes with uveitis, significant reduction of inflammation, a preserved architecture of the eye and no detectable CsA levels in the blood were recorded. Therapeutic levels of CsA within the vitreous were observed in the implanted eyes for at least 6 months. A PVA-EVA device liberating both dexamethasone and cyclosporin A was also studied. Both drugs were released for 10 weeks at similar rates to devices containing single drug without any evident clinical toxic effects. The potential therapeutic synergistic effect of this device remains to be assessed. Fluocinolone acetonide, a synthetic corticosteroid with lower solubility than dexamethasone was also studied. The low solubility should allow the extended drug relea from DDS. Vitreous implantation of PVA with silicone laminated fluocinolone acetonide yielded constant drug release over a 1 year period[101] . Based on this study, expected drug release of 2 mg and 15 mg loading devices are of 2.7 and 18.6 years, respectively. In patients with severe uveitis, intravitreal implants loaded with 0.59 mg or 2 mg of fluocinolone actetonide demonstrated marked clinical benefits. However, secondary complications were observed in these patients. Recently, results from a multi-center trial in patients with severe uveitis have shown that implanted eyes had reduced recurrence of intraocular inflammation and higher visual acuity, but cataracts, raise of intraocular pressure and retinal complications have been observed. Rod-shaped devices with drug coating made of poly butyl methacrylate and poly ethylene-co-vinyl acetate were developed and implanted in the subretinal space. These implants were used to deliver sirolimus or triamcinolone acetonide. During a follow up period of 4 weeks, although good retinal tolerance was observed, cataract and corneal edema developed in some implanted eyes. Feasibility of drug delivery with a PVA-EVA episcleral implant loaded with betamethasone was studied. This implant was localized on the sclera at the posterior pole. Betamethasone release from this device followed a zero-order kinetic profile for 4 weeks. Drug concentrations in the retina–choroid complex were higher than the needed minimal effective concentration for suppression of inflammation and above those found in the vitreous. Advantages of this implant are a less invasive implantation and a potential more sitespecific drug delivery since no drug was detectable in the aqueous humor. Drug release from these episcleral implants was studied using Gd-DTPA and magnetic resonance imaging. Further studies include a PLA intrascleral implant that was designed to sequester betamethasone. These implants were well tolerated and induced a higher drug concentration in the retina–choroid complex than in the vitreous. PLGA scleral plug loaded with FK506 reduced significantly the incidence and extent of uveitis in rabbit eyes. PGLC implants loaded with 2 mg CsA were also tested in a rabbit model of uveitis. Eyes receiving these implants showed constant drug release for at least 14 weeks along with reduction of the intraocular inflammatory processes.
Intravitreal implantation to treat Choroidal neovascular membranes (CNVM) PVA matrices loaded with various amounts of triamcinolone acetonide were implanted in rat eyes after induction of CNVM. These implants inhibited the fibrovascular disease for at least 35 days.
Intravitreal implantation of encapsulated cells for the treatment of retinal disorders
Cell encapsulation technology (ECT) for the potential treatment of eye diseases was realized using intravitreal implants made of 1.5 mm long AN69 copolymer (polyacrylonitrile-methallylsulfonate) microcapsules loaded with mouse fibroblasts secreting FGF-2 in rat eyes. The implanted cells survived for at least 90 days and their intravitreal release of FGF-2 induced a delay of photoreceptor cells degeneration in the treated eyes. The procedure was associated with changes of the retinal architecture in a few eyes. However, no rejections or FGF-2 induced tumor formation were observed in any of the treated eyes. ECT device made of polyether sulfone (PES, N99%), caprolactame (plasticizer) and polyvinylpyrrolidone antifouling)was developed and then fixed in the vitreous cavity by a titanium anchor. When loaded with cells that secrete CNTF and implanted in normal rabbit eyes, the cells remained viable and allowed constant secretion of the CNTF for the 7 weeks of the study without any adverse effects on photoreceptors function. In eyes of dogs with retinitis pigmentosa, these implants induced a dose dependent protection of photoreceptors slowing down the retinal degenerative processes in these treated eyes. A new ECT NT-501 device was designed and it includes a PES cylinder (9mm length and outer diameter of 1070 ìm) with an internal poly(ethylene terephthalate) yarn scaffold that supports the implanted cells. A titanium loop covered with biocompatible compounds was added for fixation in the vitreous. While loaded with CNTF secreting cells, this device allowed stable protein release for at least 1 year in the rabbit eye. Following these findings, a phase I clinical trial was conducted in 10 patients with vision loss from degeneration of retinal photoreceptors. In these patients’ eyes, CNTF was secreted for 6 months with improvement of the visual acuity in half of the treated eyes. A surgically related choroidal detachment was induced in one eye but no other systemic or ocular complications were observed. Examination of the implants after their removal showed that the remaining CNTF secreting cells were viable and only slightly reduced compared to the originally implanted number of cells. Mouse retinal progenitor cells (RPC) were delivered on biodegradable PLA/PLGA polymer substrates to the subretinal space of mice with retinal degeneration. Fourweeks after surgery, these polymer composite grafts induced a 10-fold increase in the number of retinal surviving cells in the treated eyes. Migration of the grafted RPCs into the host retina was also detected.
Therapeutic application of particulates drug delivery systems
Particulate drug delivery systems include nanoparticles and microparticles, nanospheres and microspheres and nanocapsules and microcapsules. The biologically active agent can be dissolved or encapsulated in the macromolecular material composing the particles. Nanoparticles can also be obtained by the formation of complexes by electrostatic forces using cationic peptides or by cationic polymers. Microcapsules are spherical entities up to several hundred microns in diameter where the drug particles or droplets are entrapped in a polymeric membrane. Microspheres are a polymer–drug combination where the drug is homogeneously dispersed in the polymeric matrix. Nanoparticles are small (b1 ìm) and divided in nanospheres where the drug is either incorporated within or attached to their surface. Nanocapsules have a central cavity surrounded by a polymeric membrane. To avoid opsonisation and recognition by the host phagocytes, the surface of the particles can be modified by pegylation. Particulate systems have the advantage of intraocular delivery by injection. Their size and polymer composition influence markedly their biological behaviour in vivo. Microparticles act like reservoir after intravitreal injection and they diffuse poorly in the vitreous gel. PLA microspheres remain in the vitreous for 1.5 months in normal rabbit eyes and for 2 weeks in vitrectomized eyes. Nanoparticles on the other hand, diffuse rapidly and are internalized in ocular tissues and cells of the anterior and posterior segment. Liposomes are vesicular lipid systems of a diameter ranging between 50 nm to a few ìm. They allow the delivery of a wide variety of drug molecules such as proteins, nucleotides, and even plasmids. Liposomes are biocompatible and biodegradable and they are composed of lipids similar to those present in biological membranes. Their membranes are stable and may undergo severe deformation without disruption. Using liposomes, hydrophilic, lipophilic or amphiphilic drugs can be encapsulated. Liposomes are readily synthesized using sterile techniques and it is possible to produce them industrially. The vesicles can also be injected under a liquid dosage form, thus a 27- or 30- gauge needle may be used for injection, even though the liposomal diameter may be greater than the lumen of the needle. Finally they can provide a convenient way of obtaining slow drug release from a relatively inert depot without changing the intrinsic characteristics of the encapsulated agents.
Injection into the anterior chamber
We have evaluated the tolerance and distribution of PLA nanospheres (20 µl, 2.7 mg/ml PLA, 0.83 µg/ml rhodamine in PBS, 180±30 nm diameter) injected in the anterior chamber of Lewis rats.Afree rhodamine solution (1.25 µg/ml BSS) was injected in control eyes. Rats were sacrificed at 1 h and 24 h after injection and the eyes processed for cryosections and immunohistology staining or for semi-thin sections and transmission electron microscopy. In other treated rats, the corneas were fixed, flat-mounted and examined with confocal microscope. Immediately after injection of the nanoparticles, the aqueous humour appeared turbid and cleared rapidly as the particles settled. At 1 h after injection, particles were mostly located at the irido-corneal angle and were internalised in epithelial cells of the iris and the ciliary body. Aggregate particles could be observed around the injection site. Unexpectedly, a large number of particles were engulfed by the corneal endothelial cells and were seen on cryosections and flat-mounted cornea (Fig. 8-1A and B). Some of the nanoparticles are also observed within the corneal stroma indicating their potential migration (or transport) through the different layers of this tissue (Fig. 8-1A). TEM observations confirmed the nanoparticles intracellular localization in endothelial cells and their stromal migration up to the epithelial basal membrane (Fig. 8-2). No toxic effect of the nanoparticles was observed after their incubation with corneas in vitro. These experiments demonstrate that nanoparticles, administered into the anterior can be used to specifically target the cornea and its various cellular layers.
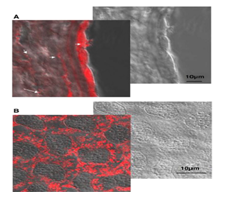
Figure 8-1: Fluorescence and confocal images of comeas at 24 h after intracameral injection of rhodamine-loaded PLA nanospheres(20 µl, 2.7 mg/ml PLA, 0.83µg/ml rhodamine in PBS, 180 ±30 nm diameter). A: fluorescence and nomaski observation of cornea sections showing the intense internalization of nonopartienles in dndothelial cells and their presence also in the stroma. B: flat mounted cornea observed using a confocal microscope and showing the presence of nanoparticles in the cytoplasm of endothelial cells.
Injection into the vitreous
Localized foreign body reaction has been observed after injection of microspheres loaded with ganciclovir, PLA or PLGA particles loaded with inert fluorochromes indicating the possible induction of an inflammatory reaction by the (co)polymers. Functionally however, the intravitreal nanoparticle injections do not affect the retinal electroretinogram responses. Due to the presence of charged groups, protein nanoparticles can also be used as a matrix for drugs which can be physically entrapped or covalently linked. Albumin nanoparticles have been tested as PDDS and showed excellent intraocular tolerance. Liposomes have widely been studied for intravitreal delivery. Interestingly, after intravitreal injection, drugs encapsulated within liposomes (antibiotics, antiviral, antifungal, antimetabolic agents…) are less toxic than the free form. The decrease of toxicity can be explained by the limited amount of drug being under a free form, directly into close contact with the tissues. This beneficial effect results from the sustained release of the molecule from the liposomes. In addition, liposomes significantly increase drug halflife in the vitreous showing their stability in this medium. However, the residence time of liposomes is shorter in infected eyes than in normal eyes. This might result from an increased rate of diffusion of liposomes through liquefied vitreous, enhanced uptake by macrophages recruited, and/or a breakdown of the blood-retinal barrier in infected eyes. Liposomes also protect poorly stable drugs from degradation as shown with phosphodiester antisense oligonucleotides and peptides.
Figure 8-2: TEM observations of corneas at 24 h after intracameral injection of PLA nanospheres (20 µl, 2.7 mg/ml PLA, 0.83 µg/ml rhodamine in PBS, 180±30 nm diameter) in the Lewis rat. A: Nanoparticles preparation observed on a formvar grid. B: Same preparation observed in the aqueous humor, collected 24 h after intracameral injection. C: Nanoparticles in the aqueous humor and at the endothelium interface. D: Particles are observed in the Descemet’s membrane, migrating from the endothelial cells(arrows). E: Particles located in the Descemet’s membrane and in the stroma(arrows). F: Anterior stroma and epithelium basal membrane with particles migrating anteriorly(arrows). G: Presence of particles in the stroma and in both sides of the epithelium basal membrane.
Potential use in proliferative vitreoretinopathy (PVR)
To reduce the PVR in rabbit eyes, PLA or PLGA microspheres loaded with 5-fluoruracil, adriamycin or retinoic acid were used. PLA microspheres containing 10 ìg adriamycin significantly reduced the rate of PVR formation. PLGA microspheres releasing retinoic acid remained in the vitreous for 40 days and also reduced the extent of PVR in treated rabbit eyes. In these experiments, the microparticles mostly settled on the inferior quadrants of the retina and induced a localized multinuclear giant cells reaction. More recently, PEI/oligonucleotide complexes forming nanoparticles were injected in the rat vitreous to evaluate the possibility to inhibit TGFâ2 expression. Interestingly, these complexes localized in retinal Müller glial cells.
Treatment of CMV retinitis
In a CMV rabbit model, a single injection of PLGA microspheres loaded with 10 mg ganciclovir was found as efficient as the direct injection of 130 µg ganciclovir every 4 days. In these experiments, the microspheres were still detected within the eye 8 weeks after their injection. Negatively charged albumin nanoparticles loaded with ganciclovir were injected in normal eyes. These particles showed a 2 weeks’ residence time within the vitreous but did not induce any noticeable inflammatory reaction in the retinal tissue and did not influence the organization of the surrounding ocular tissues. Almost 40% of the loaded drug was released within 1 h with a slower release rate during the following 10 days. These results were more recently confirmed by Irache et al., who observed in addition the possible nuclear targeting of the albumin nanoparticles when these were loaded with oligonucleotides. Akula et al evaluated in humans the potential of liposomes to treat CMV retinitis in AIDS patient. The patients received an intravitreal injection of liposomes encapsulated ganciclovir in the right eye. The left eye served as a control, receiving intravitreal free ganciclovir. The right eye showed no retinal hemorrhages or detachment; however, vision declined initially, stabilizing later. Indeed, the major clinical changes after intravitreal injection of liposomes consisted of vitreal bodies i.e. small white sparkling opacities mainly located in the lower part of the eye, vitreal condensations, and retinal abnormalities. The liposome formulations spread diffusely within the vitreous cavity and cause cloudiness, interfering with the patient’s visual acuity and the ability of the ophthalmologist to examine the fundus until complete resorption of the formulation has occurred 14–21 days after administration. Despite this drawback, weekly examination showed neither progression of the CMV retinitis nor new lesions in the eye treated with liposomes whereas eye injected with solution of ganciclovir showed reactivation of old CMV retinitis. Liposome encapsulated ganciclovir also reduced the number of intravitreal injections.
Treatment of uveitis
De Kozak et al. used polyethylene glycol-coated cyanoacrylate nanoparticles loaded with tamoxifen for the inhibition of intraocular inflammation in a rat model of experimental autoimmune uveitis (EAU). These nanoparticles induced a significant inhibition in the expression and extent of the uveitis in the treated eyes without any detectable ocular toxicity.
Delay of retinal degenerative processes
PLGA microspheres loaded with GDNF injected in the vitreous cavity of rd1-/rd1-mice delayed retinal degeneration in the treated mice eyes. Aggregation of PLGA microspheres and localized glial reaction of the retinal surface were detected in some of these treated eyes.
Injection in the subretinal space
Ogura et al. first injected rhodamine loaded PLA microspheres into the subretinal space via transvitreal approach in rabbits, and observed an RPE uptake persisting up to 4 weeks, without architectural damaging the neural retinal structure. Based upon this, Cleland et al. developed a murine model of subretinal neovascularization with the subretinal injection of PLGA microspheres releasing a recombinant human vascular endothelial growth factor[141].
Local intraocular drug delivery: Its Advantages and Disadvantages
Advantages:
Advances in materials sciences and surgical technique have allowed the development of a drug delivery system that can overcome the limitations of topical and systemic routes of administration. Implants can now be produced with a core of drug surrounded by layers of permeable and impermeable polymers. These implants can then be placed in the vitreous or subconjunctivally to deliver drug to the eye. They can also be attached to intraocular lenses placed during cataract surgery as another method of delivery.
Local delivery of drugs to the eye via intravitreal implants offers several advantages over systemic therapy. Firstly, it bypasses the blood-ocular barriers, allowing higher intraocular drug levels than could be achieved by systemic or topical administration. Secondly, it avoids many of the side effects associated with systemic therapy. This is particularly of benefit in the case of medications that may be too toxic for systemic administration but would be well tolerated by the eye. A good example is trifluoridine (Viroptic, Wellcome). Originally developed for the systemic treatment of herpetic infections, it was found to be mutagenic, carcinogenic, and teratogenic with systemic administration. Dosages as low as 1.5 mg/kg a day resulted in an increased risk of carcinomas and sarcomas in animals.However, topical administration for the treatment of herpes simplex keratoconjunctivitis and epithelial keratitis is effective and well tolerated by the eye, without the systemic exposure and increased risk of neoplasia.
Intravitreal implants also provide relatively constant drug levels in the eye. Their release rates can be carefully controlled to avoid subtherapeutic or toxic levels. This is particularly valuable when local or systemic therapy mandates intravenous administration or frequent dosing; or when patient compliance is of concern. Finally, much less drug is needed for this local drug delivery, which is especially important for new therapeutic agents that may be in short supply or extremely expensive.
Disadvantages:
There are, however, some disadvantages to intravitreal implant therapy. Local drug delivery to a single eye does not treat the contralateral eye. Furthermore, for diseases not limited to the eye, local drug delivery fails to treat extraocular disease. This is particularly the case with infectious diseases such as CMV, or ocular inflammatory diseases with systemic involvement, such as Behçet’s disease, Vogt–Kayanagi–Harada disease, and sarcoidosis. Higher intravitreal concentrations of drugs may offer a greater therapeutic effect, but may also be associated with increased ocular toxicity. Drugs that may be safe to the eye when used for a short time may prove to be toxic when allowed to maintain long standing intraocular levels. Finally, surgical placement of intravitreal implants can cause adverse effects including vitreous haemorrhage, retinal detachment, and endophthalmitis.
Conclusion
The crucial need to limit the frequency of repeated intraocular injections for the treatment of age related macular degeneration (AMD), diabetic macular oedema or the sequels of chronic intraocular inflammation has boosted the need for the development of slow release implants. To enhance the drug release efficacy and therapeutic potential, these systems have been implanted at various ocular sites. Extensive preclinical studies have evaluated different types of implants for the treatment of specific ocular diseases. As described in this review, most of the developed systems efficiently release the encapsulated active drug compounds and induce a marked therapeutic effect on experimentally induced eye diseases.However, polymer degradation in the implants may cause toxicity and the need for arduous surgical techniques has prevented the further development of these systems for routine clinical use. Non-biodegradable implants can release the encapsulated drug in a controlled manner for a long period of time. Biodegradable implants do not need to be removed and can be inserted by injection. However, the ability of the biodegradable systems (like PLGA) to release a large amount of drug is limited. Nonetheless, depending on the time course of the disease and the clinical manifestations, both types of implants can find a place in the therapeutic arsenal. Thus, combination of different systems will probably offer better potential therapeutic outcomes and have to be carefully evaluated on an individual basis. Regarding the most efficient and more yielding implantation site, accumulating knowledge indicates that the distance of implant from the diseased area is an important factor. Thus, when targeting the outer retina or the choroid, implantation within the vitreous cavity may not be the optimal option. Development of suitable transscleral or intrasscleral implants will most probably allow, in the future, for more efficient drug release to the choroid and outer retina with reduced side effects. In our laboratory, we are presently concentrating on the development of particulate drug delivery systems with specific homing characteristics and potential to controlled drug delivery. These particulate systems can be administered to the targeted ocular tissue by different routes of application without the need for surgery. Furthermore, engineering of the size and composition of these particles allows for a controllable slow release of the encapsulated drug for extended periods of time along with the potential for specific cell or tissue targeting. Also, the possibility to develop thermo or light labile particulate systems can allow for time and site control release of the encapsulated drug as desired and according to various manifestations during the disease course. Manipulation of drug release and delivery is feasible through the specific design of the implants and their chemical composition. A higher level of achievement is presently under development through miniaturization of the implants, the use of micro particulate systems and their specific characteristics such as heat or light sensitivities. The acquired knowledge and know how of the recent years nurture our hope for the potential development of efficient drug delivery systems with high therapeutic potentials and minimal undesirable side effects. It is our hope that these systems will soon find their way to the bed side allowing for more efficient treatment modalities for the presently poorly treatable blinding chronic ocular disease.