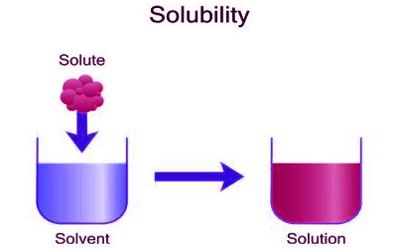
Solubility is the ability to be dissolved, especially in water. It is defined as the maximum amount of a substance that will dissolve in a given amount of solvent at a specified temperature. It is the ability of a substance (the solute), to mix into a liquid (the solvent). It is the property of a solid, liquid, or a gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent. Solubilities range widely, from infinitely soluble such as ethanol in water to poorly soluble, such as silver chloride in water.
Solubility is a property referring to the ability for a given substance, the solute, to dissolve in a solvent. It is measured in terms of the maximum amount of solute dissolved in a solvent at equilibrium.
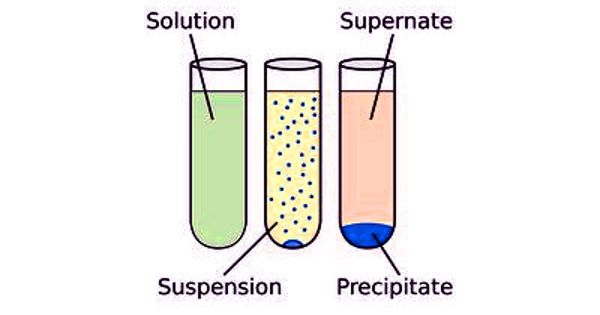
Insolubility is the inability to dissolve in a solid, liquid or gaseous solvent. The definition of solubility is the maximum quantity of solute that can dissolve in a certain quantity of solvent or quantity of solution at a specified temperature or pressure (in the case of gaseous solutes). It measures the highest amount of substance mixed into a liquid solvent while they are both at equal amounts. It occurs under dynamic equilibrium, which means that solubility results from the simultaneous and opposing processes of dissolution and phase joining. When the two mix together it is called a saturated solution. Certain substances can mix into any amount of a liquid solvent. Certain substances are soluble in all proportions with a given solvent, such as ethanol in water. This property is known as miscibility.
The solubility of a substance is an entirely different property from the rate of solution, which is how fast it dissolves. An example of this is ethanol in water. This process is better described as “miscible” (the ability to mix with one another). Solubility does not depend on the size, in fact even the large particles will eventually all dissolve. The smaller a particle is, the faster it dissolves although there are many factors to add to this generalization.
A solute is a substance that is dissolving in a solvent and is the smaller part of the solution. Solubility is the new bond formation between the solute molecules and solvent molecules. In terms of quantity, solubility is the maximum concentration of solute that dissolves in a known concentration of solvent at a given temperature. A solvent is the bigger part of a solution and is the substance that the solute dissolves in. The solubility equilibrium occurs when the two processes proceed at a constant rate. A solution is considered saturated when adding additional solute no longer increases the concentration of the solution.
Information Source: