INTRODUCTION
Pulp is the fibrous cellulosic material used in the production of paper. Wood is the principal raw material for the production of pulp; but bamboo, cotton, linen, rags, straw, bagasse, etc are also used. Chemical pulp production is the cooking of the wood raw material with chemicals.Cooking is necessary to remove the binding agents from the wood. Chemicals and heat are used to dissolve the lignin, the binding agent that holds the wood fibers together.The cooked chemical pulp, which is made up of wood fibers, is the main raw material in the production of paper and board. Both softwood and hardwood are used in the making of the chemical pulp
Chemical pulping processes such as the kraft (or sulfate) process and the sulfite process remove much of the hemicelluloses and lignin. The kraft process does less damage to the cellulose fibers than the sulphite process, thereby producing stronger fibers, but the sulfite process makes pulp that is easier to bleach. The chemical pulping processes use a combination of high temperature and alkaline (kraft) or acidic (sulphite) chemicals to break the chemical bonds of the lignin.
The material fed into the digester must be small enough to allow the pulping liquor to penetrate the pieces completely. In the case of wood, the logs are chipped and the chips screened so that what is fed to the digester is a uniform size. The oversize chips are rechipped or used as fuel, sawdust is burned. The screened chips or cut plant material (bamboo, kenaf, etc) goes to the digester where it is mixed an aqueous solution of the pulping chemicals, then heated with steam. In the kraft process the pulping chemicals are sodium hydroxide and sodium sulfide and the solution is known as white liquor. In the sulfite process the pulping chemical is a mixture of metal (sodium, magnesium, potassium or calcium) or ammonium sulfite or bisulfite.
After several hours in the digester, the chips or cut plant material breaks down into a thick porridge-like consistency and is “blown” or squeezed from the outlet of the digester through an airlock. The sudden change in pressure results in a rapid expansion of the fibers, separating the fibres even more. The resulting fiber suspension in water solution is called “brown stock”.
Brown stock washers, using countercurrent flow, remove the spent cooking chemicals and degraded lignin and hemicellulose. The extracted liquid, known as black liquor in the kraft process, and red or brown liquor in the sulfite processes, is concentrated, burned and the sodium and sulfur compounds recycled in the recovery process. Lignosulfonates are a useful byproduct recovered from the spent liquor in the sulfite process.The clean pulp (stock) can be bleached in the bleach plant using various bleaching chemicals, such as oxygen, ozone, chlorine dioxide or hydrogen peroxide or left unbleached, depending on the end use. The stock is sprayed onto the pulp machine wire, water drains off, more water is removed by pressing the sheet of fibers, and the sheet is then dried. At this point the sheets of pulp are several millimeters thick and have a coarse surface; it is not yet paper. The dried pulp is cut, stacked, bailed and shipped to another facility for whatever further process is needed.
Bleached kraft pulp and bleached sulfite pulp are used to make high quality, white printing paper. One of the most visible uses for unbleached kraft pulp is to make brown paper shopping bags and wrapping paper where strength is particularly important. A special grade of bleached sulfite pulp, known as dissolving pulp, is used to make cellulose derivatives such as methylcellulose which are used in a wide range of everyday products from laxatives to baked goods to wallpaper paste.
Mechanical pulp can also be used in paper making. Mechanical pulp is produced when the wood fibers are separated from each other through a grinding process. Mechanical pulp is used in the making of printing paper because it has good printing properties.
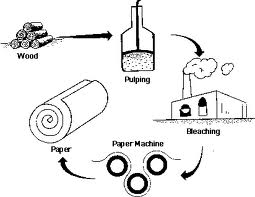
Overview on pulp and paper making process:
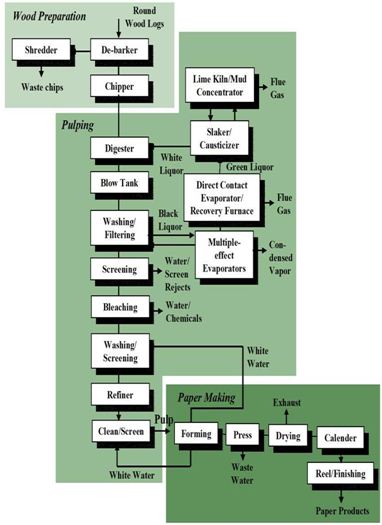
CLASSIFICATION OF PULPING PROCESS
Pulping is the process by which the bonds within the wood structure are ruptured either mechanically or chemically Generally pulping is grouped into three main categories, viz, mechanical, semichemical, and chemical.
In chemical pulping, sufficient lignin is dissolved mainly from the middle lemella to allow the fibres to separate with little, if any, mechanical action. However, a portion of lignin is retained in the fibre wall. Complete removal of lignin during pulping would result in excessive degradation of the pulp. For this reason about 3-4% lignin is normally left in hardwood chemical pulp and 4-10% in softwood chemical pulp. This is subsequently removed by bleaching if bleached pulp is desired. The different chemical pulping processes are the soda process, the sulphate or kraft process, and the sulphite process.
The semichemical pulping involves two stages: a preliminary treatment of chips with chemicals, which results in partial delignification and softening of the middle lamelia and a subsequent treatment to isolate the fibres from the softened chips. The yield range of such pulps is about 55-80%.
The mechanical pulping can be done directly from wood log, known as groundwood pulping or from chips. Mechanical pulping from chips is not used for papermaking in Bangladesh. Only groundwood process is used in the country. This process is used in newsprint mill at Khulna (KNM). Groundwood pulp is produced by pressing debarked wood bolts transversely against a revolving grooved grindstone in presence of water. The preferred wood species are light coloured and long fibred softwoods. No softwood is available in Bangladesh. Gewa wood (Excoecaria agallocha) with a mild chemical treatment before grinding is used. The yield in groundwood pulping may be upto 95%. The energy consumption varies from 1,000-1,500 kwh per ton of pulp depending on the sharpness of the grinding stone.
The highest proportion of pulp is produced by the sulphate method, followed by mechanical (including semi-chemical, thermomechanical and mechanical) and sulphite methods . Pulping processes differ in the yield and quality of the product, and for chemical methods, in the chemicals used and the proportion that can be recovered for reuse.
So, the major pulping processes are:
1.Mechanical and thermomechanical pulping process
2.Chemical pulping process:
a.Alkaline process:
(i)Kraft or sulfate pulping process
ii)Soda pulping process
b.Acid or sulfite pulping process
3.Semichemical or Neutral Sulfite Semichemical(NSSC)process
Other pulping processes are:
1.Secondary fibre pulping.
2.Rag pulping
3:Dissolving pulping
4.Solvent pulping.
Non-wood fibers are easy to be delignified by chemical pulping. It is noted that non-wood fiber are delignified much faster than wood. This can be explained by the reason of the more open structure of the fiber tissue. UNEP(1986), GIERTZ (1993) reported that the most widely used pulping method for non-wood raw materials are the soda and the sulphate processes. These processes have a relatively short cooking cycle either applied in continuous or in a batch system. This means the digester volume can be rather small. Bagasse fairly resembles hardwood in chemical composition and this is reflected in the yield which is largely the same as that of hardwood but not in cooking time. Generally speaking, further processing in the fiber production line such as screening, bleaching and drying is largely the same for the non-wood fibers as for wood.
Advantages of non-wood pulping
Non-wood raw materials are cheap. Non-wood fibers are delignified much faster than wood. The material is satisfactorily delignified and the pulp obtained is of high quality and can be easily bleached to an acceptable brightness . The cooking time of non-wood raw material is shorter than that of the hardwood. Therefore the bagasse cooking time is very short with low alkali requirements and results in a pulp with low yield.
Disadvantages of non-wood pulping
The main disadvantage of non wood pulping is the preparation of raw material .The problem lies in the preparation of fibrous raw material including collection, transportation, and storage. These are bulky material. One important thing to note is its seasonal delivery. They might deteriorate during storage. The dust loads in the handling, storage and cleaning of bagasse, bamboo and straw is high.
Comparison of typical feedstocks used in pulping | ||
Component | Wood | Nonwood |
| Carbohydrates | 65-80 % | 50-80 % |
| Cellulose | 40-45 % | 30-45 % |
| Hemicellulose | 23-35 % | 20-35 % |
| Lignin | 20-30 % | 10-25 % |
| Extractives | 2-5 % | 5-15 % |
| Proteins | < 0.5 % | 5-10 % |
| Inorganics | 0.1-1 % | 0.5-10 % |
| SiO2 | < 0.1 % | 0.5-7 % |
Wood pulp made from hardwood and softwood trees has different attributes. In Europe, hardwood accounts for 29% and softwoods 71% of wood consumption.
| Hardwood Trees | Softwood Trees | |
| Type of tree | Oaks, beeches, poplars, birches and eucalyptus | Mainly pine and spruce |
| Usage | In Europe it is mostly birches (found in Sweden, Norway, the UK and Spain) and eucalyptus (found in Portugal, Spain and Norway) that are used for papermaking. | In Europe pine is found in the UK, Norway, Finland, France, Spain, Portugal and Greece. Spruce is found in the UK, Finland, Norway and Sweden. |
| Type of fibre | Short | Long |
| Average length of fibres | 1mm | 3mm |
| Features | Achieving bulk, smoothness, opacity | Providing additional strength. Also suitable for writing and printing |
| Typical products | Writing papers, printing papers, tissue papers | Shipping containers, grocery bags, corrugated boxes |
Raw materials for pulp in Bangladesh
Wood is the principal raw material of pulp in Bangladesh.Besides wood about 5% of pulp originates from other sources mainly bamboo, bagasse, straw, etc. The coniferous woods or softwoods are the most preferred species. Although hardwoods are now increasingly being used, they are not preferred because they do not, as do many softwoods, give a uniform pulping. The chemical composition of a hardwood sometimes renders it less suitable for pulping, and sometimes its structure is so dense that pulping liquors cannot penetrate easily. Hardwoods have been considered less suitable for pulping than softwoods as they possess shorter fibre and produce less uniform pulp. Fibre length has for a long time been considered of primary importance for the quality of paper pulps.
In Bangladesh different hardwoods, viz, gamar (Gmelina arborea), shimul (Bombax ceiba), kadam (Anthocephalus chinensis), pitraj (Amora species), Koroi (Albizia species), etc, are generally used for chemical pulping. In groundwood pulping for newsprint, only gewa (Excoecaria agallocha) is used. Various exotic hardwoods, viz, akashmoni (Acacia auriculiformis), mangium (Acacia mangium), Eucalyptus, etc. are now being grown in Bangladesh. These are well-known species for pulp making.
Pulp mills in Bangladesh
There are four state-controlled pulp mills in Bangladesh.They are: Karnafuli Paper Mills (KPM), Sylhet Pulp and Paper Mills (SPPM), North Bengal Paper Mills (NBPM), and Khulna Newsprint Mills (KNM). These are run by Bangladesh Chemical Industries Corporation. Four other mills operate in the vicinity of Dhaka under private management. These are Bashundhura Paper Mills, Sonali Paper Mills, Magura Paper Mills, and Tongi Board Mills.
Among the state-controlled mills, KPM, NBPM and KNM are integrated pulp and paper mills but SPPM produces only market pulp. The four mills in the private sector use waste paper, and market and import pulp as the fibrous raw material. Waste papers are mostly imported, and only a small quantity is collected locally.
The KPM is an integrated pulp and paper mill, where the Kraft pulping process is used. Bamboo and hardwood are the fibrous raw materials. Bamboo, however, is now a scarce raw material. So the mill uses heterogeneous mixture of different hardwoods.
CHEMICALS USED IN PULP & PAPER MANUFACTURING
Common Name | Chemical Name | Chemical Formula | Used For | Specific Density | Other Characteristics |
| Agalite or Talc | Silicate of Magnesia | MgO-32%, SiO2-62% | It gives paper a greasy or soapy feel. and enables it to take a high finish. | 2.6 – 2.8 | A natural fibrous form of talc, gray in color. |
| AKD | Alkyl Ketene Dimer | Sizing | |||
| Alum | Sulfate of Alumina | Al2(SO4)3.18H2O | For alkaline sizing along with Rosin | 1.62 | |
| Albarine | Natural Sulfate of Lime | CaSO4.2H2O – 100% | A calcium salt that is used for a variety of purposes including: building materials. | 2.4 | |
| Ammonium Zirconium Carbonate (AZC) | Ammonium Zirconium Carbonate | CH2O3NH3Zr | Used as in-solubilizer, crosslinker & hardener | 1.36 | AZC is a clear, usually colorless solution, having an odor of ammonia and a pH of approximately 9.5. As a carbonate, it will react with acids, and so should not be used in coating systems below pH 7. In storage, it is stable for up to six months. |
| Anthraquinone | Anthraquinone | C14H8O2 | Added to white liquor (alkaline cooking liquor) to improve pulp yield and to increase the rate of delignification. | 1.44 | yellow crystalline powder |
| Anti-Foam/Defoamer | To prevent foam (anti-foam) or to destroy it once it has formed (defoamer). | Surface active, but highly insoluble in water. | |||
| Asbestine | Silicate of Magnesia | MgO-32%, SiO2-62% | It is used as a loading agent in paper manufacture, particularly for blotting papers and board. | 2.6 – 2.8 | A mineral compound of almost pure fibrous magnesium silicate, which possesses physical characteristics between those of talc and asbestos. |
| APE | Alkylphenol Ethoxylates | CH3(CH2)n(C6H4) (OCH2CH2)m-OH | Used as cleaning agents or surfactants | ||
| ASA | Alkenyl Succinic Anhydride | Sizing | |||
| Barium Sulfate | Barium Sulfate | BaSO4 – 100% | Used as a pigment | 4.2-4.5 | White insoluble powder. |
| Barytes | Barium Sulfate | BaSO4 – 100% | Used as filler | 4.2-4.5 | |
| Blanc Fixe | Barium Sulfate | BaSO4 – 100% | Used as a base for watercolor pigments and as a filler in paper. | 4.2-4.5 | Powdered barium sulfate |
| Casein | A milk phosphoprotein | Binder or adhesive in coating formulation | |||
| CMC | Carboxy Methyl Cellulose | Sizing | |||
| Caustic Lye or Caustic Soda or Lye | Sodium Hydroxide | NaOH | Pulping and to maintain pH | ||
| Chalk (Precipitated) | Precipitated Calcium Carbonate | CaCO3 – 100% | Filler particularly with acidic sizing coating pigment | 2.7-2.9 | High Brightness & Opacity |
| Chalk (French or Spanish) | Silicate of Magnesia | 4MgO+5SiO2+H2O; MgO – 33%; SiO2 62% | 2.6-2.9 | A soft white compact talc | |
| China Clay, Kaolin | Hydrated Silicate of Alumina | Al2O3-40%; SiO2-46%; H2O-13% | Filler, Coating | 2.4-2.7 | |
| Chlorine Dioxide | Chlorine Dioxide | ClO2 | In Pulp Bleaching | 2.86 | |
| Chlorine Gas | Chlorine | Cl2 | In Pulp Bleaching and water treatment | 2.86 | |
| Dolomite | Calcium Magnesium Carbonate | CaMg(CO3)2 | Filler, Coating | 2.86 | |
| DTPA | Diethylene Triamine Penta Acetate | Used for chelation (removal of transition metals from pulp). | |||
| EDTA | Ethylene Diamine Tetra acetic Acid | Used for chelation (removal of transition metals from pulp). | |||
| Guar Gum | Natural Polymer | Dry Strength Additive | cationic derivative | ||
| Gypsum or Mineral White or Plaster | Natural Sulfate of Lime | CaSO4.2H2O | Gypsum board, | 2.4 | |
| Hydrogen Peroxide | Hydrogen Peroxide | H2O2 | In Pulp Bleaching | 3.13-3.4 | |
| Lime | Calcium Oxide | CaO | Alkaline Pulping Process Chemical Recovery, Bleaching | 3.13-3.4 | |
| Lime Stone | Calcium Carbonate | CaCO3 | To make Lime Precipitated CaCO3 is used as Filler and in Coating | ||
| Magnesite | Magnesium Carbonate | MgCO3 -100% | 2.5 | ||
| Oxygen | Oxygen | O2 | In Pulp Bleaching | ||
| Ozone | Ozone | O3 | In Pulp Bleaching | ||
| Rosin | Abietic Acid | C19H29COOH | Sizing | ||
| Rosin Soap | Sodium Abietate | C19H29COONa | Sizing | ||
| Salt Cake | Sodium Sulfate | Na2SO4. 10H2O | Makeup chemical in sulfate pulping chemical recovery (Na2SO4. —Na2S) | 2.4 | |
| Soda Ash | Sodium Carbonate | Na2CO3 | Makeup chemical in alkaline pulping chemical recovery (Na2CO3 +Ca(OH)2 —2NaOH+CaCO3) | 2.43-2.51 | |
| Sodium Dithionite | Sodium Hydrosulfite | Na2 O4 S2 | Bleaching | 2.20 | White crystalline powder with weak sulforous odor |
| Sodium Silicate | Sodium Silicate | Na2SiO3 | In waste paper deinking for wetting, peptization, ink dispersion, peroxide stabilization. | ||
| Starch | . | Comprised of glucose units linked together by oxygen bridges called glycosides | Wet and dry end additive | ||
| Sulfur | Sulfur | S | To make HSO3 for bi-sulfite pulping | ||
| Titania | Titanium Dioxide | TiO2 | Filler to increase the opacity and brightness of paper. Used in coating also. | 3.84-4.26 |
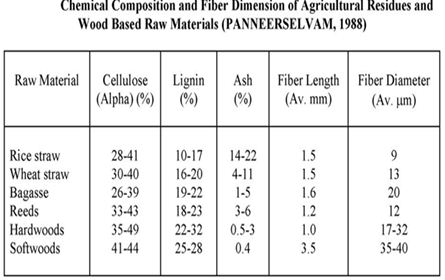
Chemical constituents and fibre morphology
Even though any ligno-cellulosic material including bamboo can be pulped with suitable methods, information on the chemical constitution and fibre morphology is important in deciding their techno-commercial suitability as well as the method of pulping. Generally, long-fibred materials with high cellulose content, low lignin, extractives and ash contents are preferred. Fibre length influences the tearing, burst and tensile strength properties of sheets. Properties like lumen and fibre diameter as well as their ratio (flexibility coefficient) and the wall thickness are known to affect the pulp strength.
Chemical constituents
Cellulose
Cellulose is the most abundant form of the naturally occurring compounds of carbon. This forms the principal component of the cell wall of all woods, straws and grasses (bamboo). As it is most frequently found in fibrous form, it has got good tensile strength, and as it is insoluble in cold and hot water, it forms an important component of pulp and paper. Cellulose is a polymeric carbohydrate, a polysaccharide with repeating units of glucose. The neighbouring glucose units are joined through carbon atoms 1 and 4.
Cellulose being relatively resistant to oxidation, lignin and other colouring matters can be removed with bleaching agents without appreciable damage to the strength of pulp. The alpha or true cellulose content of a fibrous material does not affect directly its pulpability, but the higher the alpha-cellulose content of a material, the higher the yield of fully delignified, bleached chemical and semichemical pulps.
Hemicelluloses
When wood is freed from extractives (compounds which are soluble in cold water or in neutral organic solvents) and is then carefully freed from lignin, it yields a fibrous product termed holocellulose, which represents the sum total of cellulose and other polysaccharides; the latter are usually termed hemicelluloses (or polyoses). Pulping processes remove not only lignin (imperfectly) but also some of the less resistant hemicelluloses; so, holocellulose cannot be obtained by ordinary pulping operation.
The hemicelluloses contain mainly sugar units other than glucose (such as xylose, mannose, arabinose, rhamnose, galactose, etc.). Usually the dominant unit in the hemicelluloses is xylose, but frequently mannose units are present in appreciable amounts, especially in the case of the hemicelluloses of coniferous woods. The hemicellulose fractions which contain xylose (and uronic acid) units are often termed ‘xylans’ or more loosely ‘pentosans’. Those contain mannose units linked to each other and to glucose units have been referred as ‘mannans’.
The hemicelluloses (when freed from lignin) swell more than does cellulose and are in part dispersible in water. They have adhesive properties not shared by cellulose. Whereas cellulose is fibrous, hemicelluloses are non-fibrous. Whereas cellulose is quite insoluble in cold alkali, hemicelluloses are quite soluble in dilute caustic soda. In any chemical pulping operations, some of the initial hemicelluloses are retained in the pulp. A portion of the less resistant hemicelluloses is removed during digestion, and their degradation products are then found in the spent liquors.
In the case of pulps freed from lignin by adequate and controlled bleaching, the hemicelluloses have been shown repeatedly to contribute greatly to tensile and bursting strength and to folding endurance of the pulp sheet. Both the quantity and the type of hemicelluloses in a pulp influence the pulp properties and the type of paper that can be made from such a pulp. There are certain disadvantages also about their presence. These are undesirable for dissolving grade pulp. In the case of certain bleached pulps, these are responsible for a loss in brightness of the bleached pulp on storage or aging.
Lignin
Lignin is the cementing substance between fibres and tissues and is concentrated mainly in the region of the middle lamella and imparts rigidity to wood tissue. Lignin exists in wood or bamboo as branched-chain polymer molecules. The lignin may be separated from an associated wood component either by preferentially dissolving lignin or by preferentially dissolving non-lignin components. Isolated lignins, in general, are amorphous and non-crystalline, and show definite softening points at elevated temperatures. The average molecular weight is in the range of 11,000. An important property of lignin is its capacity to absorb ultra violet light. The chemical skeleton of lignin is a phenylpropane or a “C6 – C3” or a “C9” type.
Pulping is basically and mainly a delignification process employing inorganic acids or alkalies and other compounds or organic solvents and compounds or by employing biological agents such as certain fungi which will selectively attack on lignin, causing its degradation and consequent dissolution. The amount and reactivity of lignin have a marked effect on the pulpability of the material. These differ depending upon the raw material (softwoods, hardwoods, bamboos, etc.). During most pulping reactions, components other than lignin are simultaneously removed. The character of pulp depends upon the form and amount of energy supplied for accomplishing the separation. Chemical, mechanical or a combination of the two forms of energy are utilized. In general, when chemical energy alone is supplied, completely separated fibres are obtained; whereas in mechanical and semichemical pulping (combination of mechanical and chemical processing) whole fibres, fibre bundles, damaged fibres, and fibre fragments are produced. With the various methods now available for partitioning the two forms of energy, pulps of widely diverse properties can be processed. Bleaching of pulp is also a process mainly employed for further purification of the pulp by removing the remaining portions of lignin and other colour bodies in the pulp.
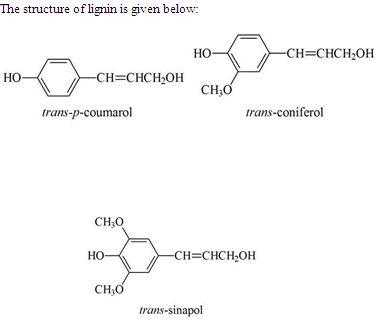
Table – Chemical constituents of pulp and paper fibre sources (%)
| Softwoods | Hardwoods | Straw | Bamboo | Cotton | |
| Carbohydrates | |||||
| α-cellulose | 38–46 | 38–49 | 28–42 | 26–43 | 80–85 |
| Hemicellulose | 23–31 | 20–40 | 23–38 | 15–26 | nd |
| Lignin | 22–34 | 16–30 | 12–21 | 20–32 | nd |
| Extractives | 1–5 | 2–8 | 1–2 | 0.2–5 | nd |
| Minerals and other inorganics | 0.1–7 | 0.1–11 | 3–20 | 1–10 | 0.8–2 |
Fibre morphology
Fibre dimensions indicate the suitability of a fibrous raw material for producing pulp. Generally, the average fibre length of soft woods, hardwoods and bamboos is 3.5, 1.3 and 2.7 mm respectively. It varies within and between species as well as within trees and due to different locations. The fibre length is roughly 100 times longer than its diameter.
The fibre length influences mainly pulp strength, the tearing resistance in particular and to a lesser extent, the burst, tensile and the fold. Properties like fibre diameter and lumen diameter, if considered individually, have no appreciable influence on pulp strength, but the cell wall thickness is known to improve the paper strength. Flexibility coefficient influences tensile strength and, to some extent, the burst strength also, while the relative fibre length influences tearing resistance. The Runkel ratio (cell wall thickness to lumen diameter) gives an indication of suitability of fibres for paper making (Runkel 1949). The values of Runkel ratio are classified into three groups.
| Runkel Group | Runkel ratio values | Relative thickness |
of cell wallRemarks for paper making 1Less than unityThinVery good 2About equal to unityMediumGood 3More than unityThickPoor
Wood, bamboo and other grasses falling under Runkel group 1 and 2 are suitable for pulp and paper making, while those falling under group 3 are of poor quality for pulping.
HEMICAL PULPING
Pulp is wood fiber that is generally used to make paper. Chemical pulp is created by a method that uses chemicals and heat to convert wood into pulp i.e. the method of converting wood chips into paper pulp for use in papermaking accomplished by chemical cooking of the chips is called chemical pulping. This process can be, and generally is, used as an alternative to mechanical pulping, which involves obtaining wood fibers by the way of a grinding process.Chemical pulp tends to be more common than mechanically derived pulp. Chemical pulping generally results in the production of paper with greater sheet strength than the paper produced by mechanical pulping.
The main purpose of chemical pulping is to remove lignin and other materials binding individual cells together and so makes fibre directly available for paper or board making. Chemical pulp is produced by combining wood chips and chemicals in large vessels known as digesters where heat and the chemicals break down the lignin.Chemical pulp is used for materials that need to be stronger or combined with mechanical pulps to give a product different characteristics. The kraft process is the dominant chemical pulping method, with sulfite process being second. Historically soda pulping was the first successful chemical pulping method.
In the past,chemical pulping was done in large batch digesters(tanks that carried a single charge at a time) which held 40-50 tons of wood chips.The chips are mixed with appropriate chemicals cooked at around 150ᵒC.The resulting pulp slurry and cooking liquor were then separated and the delignified pulp further defiberd,washed and screened for use.
For the production of chemical pulp from wood chips, following chemicals can be used:
- Caustic soda, Sodium sulfide for the Kraft process
- Sulfurous acid for the Sulfite process
- Caustic soda, Anthraquinone for the Soda pulping
KRAFT PROCESS
The kraft process (also known as kraft pulping or sulfate process) describes a technology for conversion of wood into wood pulp consisting of almost pure cellulose fibers(“kraft” is the German and Swedish word for “strength”). The process entails treatment of wood chips with a mixture of sodium hydroxide and sodium sulfide, known as white liquor, that break the bonds that link lignin to the cellulose. The kraft process differs from the sulfite process in that
(1) the cooking liquor is alkaline and therefore is less corrosive to iron and steel, so that the digesters in which the process takes place need not be lined, and
(2) the pulp produced is stronger than that produced by cooking with caustic soda alone.
A further advantage of the kraft process is its capability of digesting pine chips; the resinous components dissolve in the alkaline liquor and can be recovered in the form of tall oil, a valuable by-product. Recovery of the sodium compounds is important in the economy of the kraft process.
History
The kraft process was invented by Carl F. Dahl in 1879 in Danzig, Prussia, Germany. U.S. Patent 296,935 was issued in 1884, and a pulp mill using this technology started (in Sweden) in 1890. The invention of the recovery boiler by G.H. Tomlinson in the early 1930s, was a milestone in the advancement of the kraft process. It enabled the recovery and reuse of the inorganic pulping chemicals such that a kraft mill is a nearly closed-cycle with respect to inorganic chemicals, apart from those used in the bleaching process. For this reason, in the 1940s, the kraft process surpassed the sulfite process as the dominant method for producing wood pulp.
Impregnation
Common wood chips used in pulp production are 12–25 millimetres (0.47–0.98 in) long and 2–10 millimetres (0.079–0.39 in) thick. The chips normally first enter the presteaming where they are wetted and preheated with steam. Cavities inside fresh wood chips are partly filled with liquid and partly with air. The steam treatment causes the air to expand and about 25% of the air to be expeled from the chips. The next step is to impregnate the chips with black and white liquor. Air remaining in chips at the beginning of liquor impregnation is trapped within the chips.
The impregnation can be done before or after the chips enters the digester and is normally done below 100 °C (212 °F). The cooking liquors consist of a mixture of white liquor, water in chips, condensed steam and weak black liquor. In the impregnation cooking liquor penetrate into the capillary structure of the chips and low temperature chemical reactions with the wood begin. A good impregnation is important to get a homogenous cook and low rejects. About 40 – 60 % of all alkali consumption in the countinous processes happens in the impregnation zone.
Cooking
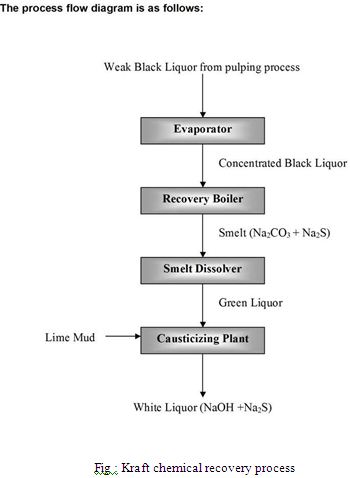
The wood chips are cooked in huge pressurized vessels called digesters. Some digesters operate in batch manner and some in continuous processes. There are several variations of the cooking processes both for the batch and the countinous digesters. Digesters producing 1,000 tonnes of pulp per day and more are common with the largest producing more than 3,500 tonnes of pulp per day. In a continuous digester the materials are fed at a rate which allows the pulping reaction to be complete by the time the materials exit the reactor. Typically delignification requires several hours at 130 to 180 °C (266 to 356 °F). Under these conditions lignin and hemicellulose degrade to give fragments that are soluble in the strongly basic liquid. The solid pulp (about 50% by weight based on the dry wood chips) is collected and washed. At this point the pulp is quite brown and is known as brown stock. The combined liquids, known as black liquor (so called because of its color), contain lignin fragments, carbohydrates from the breakdown of hemicellulose, sodium carbonate, sodium sulfate and other inorganic salts.
Kraft Digesters
Lignin removal can occur in batch digesters or continuous digesters. There are four major stages in a kraft continuous pulping process:
1. Steaming stage: The objective here is to remove air and heat the chips. This will improve subsequent chip impregnation.
2. Chip impregnation: Usually occurs between 115 and 120 C.
3. Delignification stage: Usually at 170 C. The time of delignification depends on how much lignin is to be removed.
4. Cooling/Washing stage: Separate the soluble lignin from the pulp. The lignin soluble fraction is termed black liquor and the pulp is brownstock. Must be careful to avoid precipitation of the lignin back onto the fibers.
Delignification Chemistry
The removal of both lignin and polysaccharides occurs in the process. Brownstock to be bleached will typically have lignin contents on the order of 3% for hardwods and 4-5% for softwoods. About 50% of the hemicelluloses have been removed as well as 10% of the cellulose.,polysaccharides are lost due to peeling. During the course of delignification, the reaction pH drops due to mostly acetate cleavage and peeling byproducts. All of the pulping reagents as well as the byproducts and degraded lignin are in the black liquor which is sent to chemical recovery for processing. There are 3 distinct phases of delignification:
1. Lignin Extraction: This is the fast phase of delignification and involves solubilization of wood extractives and low molecular weight lignin. The extractives are typically collected and separated into volatile (turpentine) and tall oil fractions.
2. Bulk Delignification: During bulk delignification, the majority of lignin is removed. This is a first order reaction.
3. Residual Delignification: Removal of the last vestiges of lignin is difficult and this phase is slower than the bulk phase. Care must be taken when entering into this phase as the soluble lignin can also undergo condensation reactions as described below.
Major side reactions that can occur during kraft delignification are lignin condensation, lignin precipitation onto pulp fibers, formation of smelly mercaptans and disulfide, and formation of methanol and formaldehyde. Lignin condensation will increase lignin molecular weight, and if left unchecked, this can lead to lignin precipitation onto the pulp fibers. This precipitation can also occur if the pH in the digester drops too much. Lignin solubility is higher at high pH, and too large of a decrease in pH can lead to lignin precipitation.
Recent Modifications
- Extended Delignification. One of the problems of the reduction in pH at the end of the cook is the potential of lignin precipitation onto the fibers. By adding white liquor at various locations in the digester, the pH changes are moderated. This results is better lignin removal and hence lower bleaching requirements.
- Oxygen Delignification. A treatment with oxygen and alkali directly after the delignification can remove almost half of the residual lignin. This waste stream can be combined with the black liquor for recovery. Bleach plant effluent which contains chlorine cannot be shipped to the recovery boiler.
Recovery process
The excess black liquor is at about 15 % solids and is concentrated in a multiple effect evaporator. After the first step the black liquor is about 20 – 30 % solids. At this concentration the rosin soap rises to the surface and is skimmed off. The collected soap is further processed to tall oil. Removal of the soap improves the evaporation operation of the later effects.
Table:Elemental analysis of black liquor(dry solid)sample
| Element | w/w(%) |
| C | 36.40 |
| Na | 18.60 |
| S | 4.80 |
| H | 3.50 |
| K | 2.02 |
| Cl | 0.24 |
| N | 0.14 |
| O (by diff.) | 34.30 |
| Total | 100 |
The weak black liquor is further evaporated to 65% or even 80% solids (“heavy black liquor”) and burned in the recovery boiler to recover the inorganic chemicals for reuse in the pulping process. Higher solids in the concentrated black liquor increases the energy and chemical efficiency of the recovery cycle, but also gives higher viscosity and precipitation of solids (plugging and fouling of equipment). The combustion is carried out such that sodium sulfate is reduced to sodium sulfide by the organic carbon in the mixture:
1. Na2SO4 + 2 C → Na2S + 2 CO2
This reaction is similar to Thermochemical Sulfate Reduction (TSR) in geochemistry.
The molten salts (“smelt”) from the recovery boiler are dissolved in a process water known as “weak wash”. This process water, also known as “weak white liquor” is composed of all liquors used to wash lime mud and green liquor precipitates. The resulting solution of sodium carbonate and sodium sulfide is known as “green liquor”. This liquid is mixed with calcium oxide, which becomes calcium hydroxide in solution, to regenerate the white liquor used in the pulping process through an equilibrium reaction (Na2S is shown since it is part of the green liquor, but do not participate in the reaction):
2. Na2S + Na2CO3 + Ca(OH)2 ←→ Na2S + 2 NaOH + CaCO3
Calcium carbonate precipitates from the white liquor and is recovered and heated in a lime kiln where it is converted to calcium oxide (lime).
3. CaCO3 → CaO + CO2
Calcium oxide (lime) is reacted with water to regenerate the calcium hydroxide used in Reaction 2:
4. CaO + H2O → Ca(OH)2
The combination of reactions 1 through 4 form a closed cycle with respect to sodium, sulfur and calcium and is the main concept of the so-called recausticizing process where sodium carbonate is reacted to regenerate sodium hydroxide.
The recovery boiler also generates high pressure steam which is fed to turbogenerators, reducing the steam pressure for the mill use and generating electricity. A modern kraft pulp mill is more than self-sufficient in its electrical generation and normally will provide a net flow of energy which can be used by an associated paper mill or sold to neighboring industries or communities through to the local electrical grid. Additionally, bark and wood residues are often burned in a separate power boiler to generate steam.
Although recovery boilers using G.H. Tomlinson’s invention have been in general use since the early 1930s attempts have been made to find a more efficient process for the recovery of cooking chemicals. Weyerhaeuser has operated a Chemrec first generation black liquor entrained flow gasifier successfully at its New Bern plant in North Carolina, while a second generation plant is run in pilot scale at Smurfit Kappa’s plant in Piteå, Sweden.
Blowing
The finished cooked wood chips are blown by reducing the pressure to atmospheric pressure. This releases a lot of steam and volatiles. The steam produced can then be used to heat the pulp mill and any excess used in district heating schemes or to drive a steam turbine to generate electrical power. The volatiles are condensed and collected, in the case of northern softwoods this consists mainly of raw turpentine.
Screening
Screening of the pulp after pulping is a process whereby the pulp is separated from large shives, knots, dirt and other debris. The accept is the pulp. The material separated from the pulp is called reject.
The screening section consists of different types of sieves (screens) and centrifugal cleaning. The sieves are normally set up in a multistage cascade operation because considerable amounts of good fibres can go to the reject stream when trying to achieve maximum purity in the accept flow.
The fiber containing shives and knots are separated from the rest of the reject and reprocessed either in a refiner and/or is sent back to the digester. The content of knots are typically 0.5 – 3.0% of the digester output, while the shives content are about 0.1- 1.0%.
Washing
The brown stock from the blowing goes to the washing stages where the used cooking liquors are separated from the cellulose fibers. Normally a pulp mill has 3-5 washing stages in series. Washing stages are also placed after oxygen delignification and between the bleaching stages as well. Pulp washers use counter current flow between the stages such that the pulp moves in the opposite direction to the flow of washing waters. Several processes are involved: thickening / dilution, displacement and diffusion. The dilution factor is the measure of the amount of water used in washing compared with the theoretical amount required to displace the liquor from the thickened pulp. Lower dilution factor reduces energy consumption, while higher dilution factor normally gives cleaner pulp. Thorough washing of the pulp reduces the chemical oxygen demand (COD).
Several types of washing equipment are in use:
- Pressure diffusers
- Atmospheric diffusers
- Vacuum drum washers
- Drum displacers
- Wash presses
Bleaching
In a modern mill, brownstock (cellulose fibers containing approximately 5% residual lignin), produced by the pulping is first washed to remove some of the dissolved organic material and then further delignified by a variety of bleaching stages.
In the case of a plant designed to produce pulp to make brown sack paper or linerboard for boxes and packaging, the pulp does not always need to be bleached to a high brightness. Bleaching decreases the mass of pulp produced by about 5%, decreases the strength of the fibers and adds to the cost of manufacture
Process chemicals
Process chemicals are added to improve the production process:
- Impregnation aids: Surfactants may be used to improve impregnation of the wood chips with the cooking liquors.
- Anthraquinone is used as a digester additive. It works as a redox catalyst by oxidizing cellulose and reducing lignin. This protects the cellulose from degradation and makes the lignin more water soluble.
- An emulsion breaker can be added in the soap separation to speed up and improve the separation of soap from the used cooking liquors by flocculation.
- Defoamers remove foam and speed up the production process. Drainage of washing equipment is improved and gives cleaner pulp.
- Dispersing agents and complexing agents are keeping the system cleaner and reduce the need for maintenance stops.
- Fixation agents are fixating finely dispersed potential deposits to the fibers and thereby transporting it out of the process.
Comparison with other pulping processes
Pulp produced by the kraft process is stronger than that made by other pulping processes and maintaining a high effective sulfur ratio or sulfidity is important for the highest possible strength. Acidic sulfite processes degrade cellulose more than the kraft process, which leads to weaker fibers. Kraft pulping removes most of the lignin present originally in the wood whereas mechanical pulping processes leave most of the lignin in the fibers. The hydrophobic nature of lignin interferes with the formation of the hydrogen bonds between cellulose (and hemicellulose) in the fibers needed for the strength of paper (strength refers to tensile strength and resistance to tearing).
Kraft pulp is darker than other wood pulps, but it can be bleached to make very white pulp. Fully bleached kraft pulp is used to make high quality paper where strength, whiteness and resistance to yellowing are important.
The kraft process can use a wider range of fiber sources than most other pulping processes. All types of wood, including very resinous types like southern pine, and non-wood species like bamboo and kenaf can be used in the kraft process.
Byproducts and emissions
The main byproducts of kraft pulping are crude sulfate turpentine and tall oil soap. The availability of these are strongly dependent on wood species, growth conditions, storage time of logs and chips and the mills process Pines are the most extractive rich woods. The raw turpentine is volatile and is distilled of the digester, while the raw soap is separated from the spent black liquor by decantation of the soap layer formed on top of the liquor storage tanks. From pines the average yield of turpentine is 5–10 kg/t pulp and of crude tall oil is 30–50 kg/t pulp.
Various byproducts containing hydrogen sulfide, methyl mercaptan, dimethyl sulfide, dimethyl disulfide, and other volatile sulfur compounds are the cause of the malodorous air emissions characteristic for pulp mills utilizing the kraft process. Outside the modern mills the odour is perceivable only during disturbance situations, for example when shutting the mill down for maintenance break. This is due to practiced collection and burning of these odorous gases in the recovery boiler along with black liquor. The sulfur dioxide emissions of the kraft pulp mills are much lower than sulfur dioxide emissions from sulfite mills. In modern mills where high dry solids are burned in the recovery boiler hardly any sulfur dioxide leaves the boiler. This is mainly due to higher lower furnace temperature which leads to higher sodium release from the black liquor droplets that can react with sulfur dioxide forming sodium sulfate.
Pulp mills are almost always located near large bodies of water due to their former substantial demands. Delignification of chemical pulps released considerable amounts of organic material into the environment, particularly into rivers or lakes. The wastewater effluent can also be a major source of pollution, containing lignins from the trees, high biological oxygen demand (BOD) and dissolved organic carbon (DOC), along with alcohols, chlorates, heavy metals, and chelating agents. Reducing the environmental impact of this effluent is accomplished by closing the loop and recycling the effluent where possible, as well as employing less damaging agents in the pulping and bleaching processes. The process effluents are treated in a biological effluent treatment plant, which guarantees that the effluents are not toxic in the recipient.
Advantages of Kraft process
- Any kinds of wood can be used.
- Substantial amount of bark can be tolerated.
- Cooking time is less.
- The pulp has excellent strength.
- Recovery process of black liquor is well established.
- Valuable by-products are obtained.
Disadvantages of Kraft process
- Contain bad smell of organic sulfur compounds
- High investment cost.
- High cost of bleaching.
- Poor color of unbleached pulp
SODA PULPING PROCESS
Soda pulping is a chemical process for making wood pulp with sodium hydroxide as cooking chemical. In the Soda-AQ process, anthraquinone (AQ) may be used as a pulping additive to decrease the carbohydrate degradation. The soda process gives pulp with lower tear strength than other chemical pulping processes (sulfite process and kraft process), but has still limited use for easy pulped materials like straws and some hardwoods.
History
The first chemical process for reducing wood to pulp was the soda process, so-named because it uses caustic soda as the cooking agent. This process was developed in 1851 by Hugh Burgess and Charles Watt in England, who secured an American patent in 1854. The first mill to use this process was built on the Schuylkill River near Philadelphia, and began operations in 1855 under the direction of Burgess, who served as manager of the mill for nearly forty years.
By 1883 it was reported that the Penobscot Chemical Fiber Co. at Great Works, Maine, was completing one of the largest pulp mills in the country. It was to have five digesters with a total capacity of 20 tons of pulp per day, and was said to cost about $320,000. The entire output of this mill was, therefore, distinctly less than that of a single digester in a modern plant.
Pulping
The success of the soda process depends on the solubility of certain constituents of the wood in the alkaline cooking solution, and the decomposition of other constituents causing the formation of acid products, which are at once brought into solution as sodium salts. Both of these actions neutralize the alkali and make it useless for further work until it is regenerated. The degradation or destruction of that portion of the wood which is dissolved is fairly complete, and it has not proved possible to prepare from it any useful by-products, though a considerable amount of sodium acetate, and a small amount of sodium formate are known to be present in solution.
The action of caustic soda on wood is very appreciable at ordinary temperatures, and is much more rapid and complete as the temperature is raised. It is, therefore, customary to cook the wood in the form of chips in closed digesters at high temperature and pressure. The digesters in which the cooking is accomplished have undergone considerable evolution. At first they were small globular or cylindrical rotary vessels into which the chips and cooking liquor were charged through a manhole, while the steam for cooking entered through the trunnion as the vessel was turning. Such digesters were of relatively small capacity and much time was lost in filling, and in blowing down pressure so the manhole lid could be removed, and in emptying the charge by rotating the digester. However, they did have the advantage of very complete mixing, or circulation, during the cook so that all parts of the charge were equally treated.
When these rotary digesters had reached a size beyond which their construction was impractical, vertical, stationary digesters came into use. These were at first of not much greater capacity than the rotary type, but have gradually increased in size until a digester holding 15 cords of wood is not at all unusual. Such a digester generally consists of a tall cylindrical section with a cone-shaped bottom and a dish-shaped top. In this the steam enters through numerous openings in a pipe encircling the digester, and the liquor for circulation is taken from under a false bottom. A 15-cord digester of this type would have a capacity of about 3300 cubic feet, and might be about 11 feet 10 inches in diameter and 27 feet high in the cylindrical part with an additional inverted cone 7 feet high at the bottom. The older types of digesters were built of riveted plates and it was found difficult to keep them from leaking; modern ones are electrically welded with much more satisfactory results. Since the cooking liquor has almost no action on iron and steel it is not necessary to give the digesters any protective lining, but because there is no inner lining an insulating outer covering of about 3 inches is required, both for steam economy and operating comfort.
Vertical digesters are filled with chips through a top opening which can be closed with a heavy lid, held down by swing bolts. The chips are fed in from hoppers overhead or are brought to the digesters directly on belt conveyors. The cooking liquor, which is essentially a solution of caustic soda, is often run in at the same time as the chips. The lid is then fastened down and cooking is started by blowing live steam into the bottom of the digester. This is continued until the desired temperature and pressure are reached, after which these conditions are maintained as long as is considered necessary to complete the cooking of the chips. During this period, and especially at first, a valve is opened at the top of the digester to allow the escape of air which was present in the chips and in the digester. This “relief,” as it is called, aids the circulation of liquor in the digester and gives more uniform cooking conditions throughout the charge. Uniformity is sometimes insured by pumping the liquor from the bottom of the digester and discharging it on top of the chips. A variation of this procedure is to pump the cooking liquor through an exchange heater, instead of blowing steam directly into the charge; this prevents dilution of the liquor by condensed steam and permits a stronger waste liquor to go to the evaporators in the soda recovery plant, but the time for heating is longer.
The cooking conditions vary to some extent in different mills, but the important factors are the same in all. The percentage of caustic soda on the dry weight of the wood has the greatest influence on the quality of the fiber. Below a certain amount, the cooked chips are raw and dark colored, and the fiber is full of “shives,” which are small bundles of partially cooked fibers which will not break apart readily. Such fiber bleaches only with great difficulty. If too large an amount of caustic is used the wood is overcooked, the fiber likely to be tender, and the yield is somewhat reduced. The proper amount of soda to use depends somewhat on the kind of wood used, and in general it is greater for coniferous than for deciduous woods. For the latter the amount of caustic soda used will range from about 18 to 22 per cent of the dry wood, and for coniferous wood it may be as much as 26 per cent. Not all of this is used up in the cooking process, as the liquor remaining at the end of a cook may contain as much as 10-15 per cent of the caustic soda originally present. This is not a complete waste, as it appears to perform the necessary function of preventing the dissolved organic matter from separating out and again contaminating the fibers.
The other important factors in cooking are the temperature, which, of course, depends on the steam pressure used, and the time during which it is maintained. These are to some extent inter-changeable, a longer cook at lower temperature giving about the same results as a shorter one at higher temperature. Cooking can be carried out successfully at as low a steam pressure as 70 pounds per square inch if the caustic is increased above the normal, but under average mill conditions a steam pressure of 110 pounds per square inch (corresponding to a temperature of 335°F.) is common, and some mills even go as high as 130 pounds per square inch (354°F.). The time during which this temperature is maintained varies considerably in different mills, and is often more than is actually needed to pulp the wood completely. It is difficult to set an average figure, but with 22 per cent caustic and a steam pressure of 110 pounds per square inch, four hours at full pressure should be ample to produce well cooked fiber from nearly any of the deciduous woods.
When a digester charge is considered to be sufficiently cooked it is discharged through a valve in the bottom, and is blown by the pressure in the digester through a pipe into a separator which collects the fiber while allowing the steam to pass off. The escaping steam usually goes to some form of device which permits its utilization in heating water to wash the pulp. Unfortunately there is no rapid way to determine when the chips in a digester are cooked, so the time at which a cook should be blown has to be established by previous experience. Occasionally a charge will not blow out clean, and this can only be discovered by removing the lid and inspecting the inside of the digester. If much of the charge remains it is sometimes necessary to replace the lid, again steam to pressure-possibly after adding water or black liquor-and re-blow. The cause of such poor blows is not always easy to discover but they can be caused by local poor circulation or by the use of too little caustic soda to give complete cooking of the chips.
For a digester holding about 15 cords of poplar wood the data for a cook would be about as follows:
- Weight of wood charged, dry basis 34,500 lbs
- Caustic soda used 7,600 lbs.
- Volume of cooking liquor 11,200 gals.
- Time for filling with chips 20 min
- Steaming to pressure 120 min.
- Time at full pressure 180 min.
- Time for discharging 20 min.
The fibers which are collected by the separator, or blow tank, are thoroughly saturated with the liquor in which over half the weight of the original wood has been dissolved. The liquor is called “black liquor” because of its color and its removal from the fiber is essential because the caustic soda has to be recovered for reasons of economy, and because even small amounts of it remaining in the pulp make its bleaching difficult or impossible. Washing the fiber is accomplished in several ways, the oldest of which is by placing it in open tanks with perforated bottoms, through which the black liquor drains. The stock in the tank is first flooded with weak black liquor, which forces the strong black liquor ahead of it by downward displacement. When the liquor draining away reaches a certain strength-generally determined by specific gravity-water is substituted for the weak black liquor and the washing continued. This wash is collected to be used again as weak black liquor on the next tank of stock. When this reaches a low strength, beyond which it does not pay to evaporate it for soda recovery, it is allowed to go to the sewer, and the washing is continued until the color of the water flowing away indicates that the fiber is sufficiently clean for bleaching.
Another, and more modern method of washing is to dilute the stock from the blow pit with strong black liquor and pump it to continuous rotary filters. The first of these removes as much black liquor as possible; then the stock discharged from it is again diluted either with weak black liquor or water, and goes to a second filter similar to the first. Washing in this way saves much floor space and time and is said to send a stronger black liquor to the recovery plant while using less wash water, and washing the fiber more completely.
After completing the washing, the pulp goes to a chest where soaking in water permits a little more soda to be removed by diffusion from the inside of the fibers. It then passes through “knotters,” which are usually centrifugal screens with openings, and then through other screens with finer openings. These final screens may be either centrifugal or flat types, and the size of the openings must be selected according to the type of fiber being made, longer fibers requiring larger openings than short ones. After the final screening the fiber is ready for the bleaching operation, if white pulp is being made, or for making into paper if the product is unbleached or brown in color.
Recovery of soda chemicals
The recovery of the soda used by this process is a very vital factor in the cost of the fiber-without good recovery the process could not be operated at a profit. Recovery involves
(1) the evaporation of the black liquor to such a concentration that the organic matter present will burn,
(2) the dissolving of the resulting soda after burning, and
(3) the conversion of the soda to caustic soda by treatment with lime.
Evaporation is universally carried out in multiple “effect” evaporators. These consist of a series of large vessels fitted with tubes through which the liquor passes, and a steam jacket surrounding the tubes. Evaporators operate on the principle that the boiling point of the liquor is lowered by reducing the pressure under which it boils. By this means the only heat required from outside sources is the steam entering the jacket of the first vessel, or “effect.” After this the steam formed by the evaporation of the liquor in that “effect” boils the liquor in the next, and so on through the series, which may include five or more “effects.” Operating on black liquor from the soda process such an evaporator will raise the solid content of the liquor from about 16 per cent on entering to about 60 per cent in the discharge. This is not quite concentrated enough to support its own combustion, so some further evaporation is necessary.
The older method of recovery involved the burning of the black liquor in rotary furnaces, known as black ash furnaces. In this the strong black liquor from the evaporators enters at the back of the brick-lined furnace and gradually works forward to the discharge end as the furnace revolves. Some heat has to be supplied by burning wood or coal in the traverse furnace, which is a fire box on wheels so that it can be drawn back to enable repairs to be made to the furnace proper. In passing through the furnace the black liquor becomes more concentrated and finally burns, dropping into the ash car in a red-hot, or sometimes flaming, condition. This “black ash” then has to be leached with water to dissolve the soda, the solution of which is then separated from the residue of carbon, known as black ash waste. The soda ash solution goes to the causticizing plant for con-version to caustic soda, but the black ash waste finds no practical use and so has to be disposed of as a waste. It is chiefly carbon with small amounts of soda and mineral impurities.
Modern recovery method
Modern recovery methods utilize tall, rectangular furnaces very similar to present-day steam boilers which use oil or powdered coal as fuel. The walls are of water-tubes, lightly coated with a plastic refractory for protection of the metal. Once such a furnace is brought to the right temperature by burning oil, or other fuel, black liquor may be sprayed in and will burn with no further fuel consumption provided it has been evaporated to a sufficiently high solids content. Since this concentration is not usually reached in the ordinary multiple-effect evaporators the final concentration to 70-80 per cent solids is carried out in a single-effect, forced-circulation evaporator, using high pressure steam, or in a disc evaporator, in which revolving discs carry the liquor up into the hot gases passing from the furnace. In furnaces of this type practically all of the organic material in the black liquor is burned out and the soda flows from the bottom of the furnace in a red-hot, molten stream, through a water-cooled spout into a tank of water, in which it is dissolved. This type of recovery furnace utilizes the heating value of the black liquor much more fully than the black ash furnaces, even though the latter have boilers attached to absorb the heat, and large amounts of steam at high pressure are produced. The much higher temperature in the newer type furnaces causes a considerable loss of soda by volatilization and unless some form of precipitator or scrubber is provided to take this soda out of the flue gases very appreciable financial loss is suffered, and a neighborhood nuisance is created.
In order to complete the cycle of operations the soda ash recovered from the burning of the black liquor has to be converted to caustic soda. This is accomplished by treating the soda ash solution with slaked lime which causes the precipitation of calcium carbonate and the formation of caustic soda in solution. Since this reaction is never complete, there is always more or less soda ash in the cooking liquor. This is not desired, but cannot be avoided when making caustic soda in this way. It does no particular harm in the cooking process but is just so much excess material which has to be carried all through the system, and which is subject to the losses which take place all along the line. The causticizing of the soda ash is carried out either by a batch operation or in continuous systems, the latter being the more modern and preferred. The caustic soda is separated from the calcium carbonate, or lime sludge, as it is called, either by settling or by continuous filters, and the sludge is washed with water to remove as much as possible of the caustic soda. In some plants the lime sludge is reburned in rotary kilns to recover the lime for reuse.
During all these operations there is a loss of soda, and this must be kept at a minimum in order to hold the cost of fiber at a reason-able figure. Such losses are caused by incomplete washing of the fiber, by the soda content of the final wash water which it does not pay to evaporate, by the soda in the lime sludge, by volatilization from the recovery furnaces, and by any leaks or spillages all along the line. In the very best of mills the percentage of soda which is recovered for reuse is said to be 95, but this is seldom reached and 84 per cent would be much nearer the actual figure, especially for the older mills.
The use to which soda fiber is put depends on the kind of wood from which it is prepared. If it is made from coniferous woods the fibers are long and can be used in papers requiring good strength. Most soda fiber is made from hardwoods and is classed as short, hence gives paper of low strength, especially tearing strength, if used alone. When used in mixture with long fibers, soda fiber aids in producing a smooth surface and giving a well formed, uniform appearance on looking through the sheet.
Pulp characteristics
The pulp as blown from the digester is a light greyest brown color.It is almost always bleached before using, since the paper grades in which it is customarily used are white.
Soda pulping as solution for silicate scaling
Many grasses, bagasse, bamboo and some tropical hardwoods contain much silicates that may cause sodium aluminum silicate scales. Moderate amounts of silicates can be controlled with purging lime mud or lime kiln ash. Silicate removal from green liquor in a soda mill can be achieved by lowering the pH of the liquor with CO2-containing flue gases from the lime kiln or other sources. No commercial silicate removal system is available for the kraft process, but it can handle the small amounts of silicates from northern woods.
Simulation of tagasaste pulping using soda-anthraquinone
In this work, published experimental result data of the pulping of tagasaste (Chamaecytisus proliferus L.F.) with soda and anthraquinone (AQ) have been used to develop a model using a neural network. The paper presents the development of a model with a neural network to predict the effects that the operational variables of the pulping reactor (temperature, soda concentration, AQ concentration, time and liquid/solid ratio) have on the properties of the paper sheets of the obtained pulp (brightness, traction index, burst index and tear index). Using a factorial experimental design, the results obtained with the neural network model are compared with those obtained from a polynomial model. The neural network model shows a higher prediction precision that the polynomial model.
Advances in the chemical utilization of alkali lignin
Large quantities of alkali lignin are produced as by-products by the South African pulping industry. The potential utilization of industrial soda/anthraquinone (soda/AQ) eucalyptus, kraft pine and soda bagasse lignin was subsequently investigated. The molecular mass distributions of the three lignins were similar when determined by high pressure gel permeation chromatography (HP-GPC). The quantitative and qualitative occurrence of various low molecular mass lignin fragments in the different spent liquors, on the other hand, indicated that the three lignins have substantial chemical differences. Analysis of the purified degraded lignins by NMR, methoxyl content determinations, elemental analysis, carbohydrate content determinations etc., quantified various of the chemical properties of the lignin. The properties of the three lignins were ultimately used.
Chemical characterization of pitch deposits produced in the manufacturing of high-quality paper pulps from hemp fibers
Pitch deposits are extracted with acetone, and the extracts analyzed by gas chromatography (GC) and gas chromatography/mass spectrometry (GC/MS). Acetone extracts (15-25% of pitch deposits) are constituted by the defoamers used at the mill and by lipophilic extractives from hemp fibers. Acetone-insoluble residues (75-85% of pitch deposits) are analyzed by pyrolysis-GC/MS in the presence and absence of tetramethylammonium hydroxide. These residues are constituted by salts of fatty acids (arising from hemp fibers) with calcium, magnesium, aluminum and other cations that are identified in the deposits. It is concluded that inappropriate use of defoamer together with the presence of multivalent ions seemed to be among the causes of hemp extractives deposition in the pitch problems reported here.
Soda-anthraquinone pulping of palm oil empty fruit bunches and beating of the resulting pulp
The influence of soda-anthraquinone pulping variables (temperature, time and soda concentration) and beating (number of PFI beating revolution) of palm oil empty fruit bunches (EFB) on the resulting paper sheets is studied, with a view to identifying the optimum operating conditions. Equations were derived that reproduced the properties of the paper sheets with errors less than 10-12% in 90-95% of cases. An optimum compromise is found as regards operating conditions (15% soda, 170ᵒC, 70min and 2400 number of PFI beating revolutions) that provided paper properties departing by less than 12% from their optimum values (59.63Nm/g tensile index, 4.48% stretch, 4.17kN/g burst index and 7.20mNm2/g tear index), and a beating grade of 47.5degreeSR, acceptable to obtain paper sheets.
SULFITE PROCESS
The sulfite process produces wood pulp which is almost pure cellulose fibers by using various salts of sulfurous acid to extract the lignin from wood chips in large pressure vessels called digesters. The salts used in the pulping process are either sulfites (SO32−), or bisulfites (HSO3−), depending on the pH. The counter ion can be sodium (Na+), calcium (Ca2+), potassium (K+), magnesium (Mg2+) or ammonium (NH4+).
History
The use of wood to make pulp for paper began with the development of mechanical pulping in Germany by F.G. Keller in the 1840s. Chemical processes quickly followed, first with J. Roth‘s use of sulfurous acid to treat wood, followed by Benjamin Chew Tilghman‘s US patent on the use of calcium bisulfite, Ca(HSO3)2, to pulp wood in 1867. Almost a decade later in 1874 the first commercial sulfite process|sulfite pulp mill was built in Sweden. It used magnesium as the counter ion and was based on work by Carl Daniel Ekman.
By 1900 sulfite pulping had become the dominant means of producing wood pulp, surpassing mechanical pulping methods. The competing chemical pulping process, the sulfate or kraft process was developed by Carl F. Dahl in 1879 and the first kraft mill started (in Sweden) in 1890. The invention of the recovery boiler by G.H. Tomlinson in the early 1930s allowed kraft mills to recycle almost all of their pulping chemicals. This, along with the ability of the kraft process to accept a wider variety of types of wood and produce stronger fibers made the kraft process the dominant pulping process starting in the 1940s. Sulfite pulps now account for less than 10% of the total chemical pulp production and the number of sulfite mills continues to decrease.
Magnesium was the standard counter ion until calcium replaced it in the 1950s. Sulfite pulping was the dominant process for making wood pulp until it was surpassed by the kraft process in the 1940s. Sulfite pulps now account for less than 10% of the total chemical pulp production.
Processes involved in sulfite pulping
Pulping liquor preparation
The pulping liquor for most sulfite mills is made by burning sulfur with the correct amount of oxygen to give sulfur dioxide, which is then absorbed into water to give sulfurous acid.
S + O2 → SO2
SO2 + H2O ⇌ H2SO3
Care must be taken to avoid the formation of sulfur trioxide since it gives undesired sulfuric acid when it is dissolved in water.
2 SO2 + O2 → 2SO3
SO3 + H2O ⇌ H2SO4
Sulfuric acid is undesirable since it promotes hydrolysis of cellulose without contributing to delignification.
The cooking liquor is prepared by adding the counter ions as hydroxides or carbonates. The relative amounts of each species present in the liquid depend largely on the relative amounts of sulfurous used. For monovalent (Na+, K+ and NH4+) hydroxides, MOH:
H2SO3 + MOH → MHSO3 + H2O
MHSO3 + MOH → M2SO3 + H2O
For divalent (Ca2+, Mg2+) carbonates, MCO3:
MCO3 + 2H2SO3 → M(HSO3)2 + CO2 + H2O
M(HSO3)2 + MCO3 → 2 MSO3 + CO2 + H2O
Pulping
Sulfite pulping is carried out between pH 1.5 and 5, depending on the counterion to sulfite (bisulfite) and the ratio of base to sulfurous acid. The pulp is in contact with the pulping chemicals for 4 to 14 hours and at temperatures ranging from 130 to 160 °C (266 to 320 °F) again depending on the chemicals used.
Most of the intermediates involved in delignification in sulfite pulping are resonance-stabilized carbocations formed either by protonation of carbon-carbon double bonds or acidic cleavage of ether bonds which connect many of the constituents of lignin. It is the latter reaction which is responsible for most lignin degradation in the sulfite process. The electrophilic carbocations react with bisulfite ions (HSO3–)to give sulfonates.
R-O-R’ + H+ → R+ + R’OH
R+ + HSO3– → R-SO3H
The sulfite process does not degrade lignin to the same extent that the kraft process does and the lignosulfonates from the sulfite process are useful byproducts.
Chemical recovery
The spent cooking liquor from sulfite pulping is usually called brown liquor, but the terms red liquor, thick liquor and sulfite liquor are also used (compared to black liquor in the kraft process). Pulp washers, using countercurrent flow, remove the spent cooking chemicals and degraded lignin and hemicellulose. The extracted brown liquor is concentrated, in multiple effect evaporators. The concentrated brown liquor can be burned in the recovery boiler to generate steam and recover the inorganic chemicals for reuse in the pulping process or it can be neutralized to recover the useful byproducts of pulping. Recent developments in Chemrec’s black liquor gasification process, adapting the technology to use in the sulfite pulping process, could make second generation biofuels production an alternative to the conventional recovery boiler technology.
The sulfite process can use calcium, ammonium, magnesium or sodium as a base.
Calcium-based
Initially calcium was the preferred base because it was cheap and convenient to use as it obtained as inexpensive calcium carbonate .However,no recovery system is available for this base,so most calcium base mills either have ceased operation or have converted to sodium,magnesium or ammonium for which recovery systems are available.For calcium based liquor,the gas is passed through towers packed with limestone with water flowing down through the tower.
Because of the limited solubility of calcium bisulfites Ca(HSO3)2,the pH of the liquor is very low(about 2) and free sulfurous acid is present.This usually is called acid sulfite process.Because solution of Na,Mg and NH4 bisulfite are all soluble at pH 4.5,the current practice is to pulp at higher pH,which is usually called bi-sulfite pulping.Extremely long cooking times(7-10h) are necessary with acid sulfites where as 4-5h is sufficient with bi-sulfites.
Ammonia-based
Ammonia-based processes do not allow recovery of the pulping chemicals since ammonia or ammonium salts are oxidized to nitrogen and nitrogen oxides when burned.As long as aqueous NH3 remains low ion price,this process will be attractive.
Magnesium-based
The recovery process used in magnesium-based sulfite pulping the “Magnefite” process is well developed. The concentrated brown liquor is burned in a recovery boiler, producing magnesium oxide and sulfur dioxide, both of which are recovered from the flue gases. Magnesium oxide is recovered in a wet scrubber to give a slurry of magnesium hydroxide.
MgO + H2O → Mg(OH)2
This magnesium hydroxide slurry is then used in another scrubber to absorb sulfur dioxide from the flue gases producing a magnesium bisulfite solution that is clarified, filtered and used as the pulping liquor.
Mg(OH)2 + 2 SO2 → Mg(HSO3)2
Sodium-based
Sodium base is the easiest to prepare(NaCO3 or NaOH usually is used as the make up chemicals) and gives the highest quality pulp; however,recovery processes though available,are complicated and expensive.Sodium-based processes use a recovery system similar to that used in the kraft recovery process, except that there is no “lime cycle”.
Comparison of bases for sulfite pulping
| Property | Calcium | Magnesium | Sodium | Ammonium |
| SO2 absorption system | Complex | Relatively simple | Simple | Simple |
| pH range for digestion | <2 | <2 | 0-14 | 0-14 |
| Rate of pulping | Intermediate | Intermediate | Slow | Fast |
| Level of screening | Moderate | Moderate | Low | Slow |
| Scaling tendency | High | Moderate | Low | Slow |
| Ease of liquor incineration | Difficult | Simple | Complex | Simple |
| Recovery of base | No | Yes | Yes | No |
| Recovery of SO2 | No | Yes | Yes | Yes |
Advantages of sulfite process:
· Higher yield of same kappa number
· Higher unbleached brightness
· Better bleachibility
· Better beatibility
Disadvantages of sulfite process:
· Limited use of pulpwood species
· Expensive pulpwood e.g.spruce
· Low strength properties,especially tear
· Low opacity
· Water pollution problems
· Longer cooking time
Applications
The sulfite process is acidic and one of the drawbacks is that the acidic conditions hydrolyze some of the cellulose, which means that sulfite pulp fibers are not as strong as kraft pulp fibers. The yield of pulp (based on wood used) is higher than for kraft pulping and sulfite pulp is easier to bleach.
Commodity
Sulfite pulp remains an important commodity, especially for specialty papers and as a source of cellulose for non-paper applications. It is used to make fine paper, tissue, glassine. and to add strength to newsprint.
Byproducts
Sulfite pulping is generally less destructive than kraft pulping, so there are more usable byproducts.
Lignosulfonates
Chief among sulfite process byproducts are lignosulfonates, which find a wide variety of uses whereas relatively inexpensive agent is needed to make a water dispersion of a water-insoluble material. Lignosulfonates are used in tanning leather, making concrete, drilling mud, drywall and so on.
Oxidation of lignosulfonates was used to produce vanillin (artificial vanilla), and this process is still used by one supplier (Borregaard, Norway) while all North American production by this route ceased in the 1990s.
Other byproducts
Acid hydrolysis of cellulose during sulfite pulping produces monosaccharides, predominantly mannose, which can be fermented to produce ethanol.
DISSOLVING PULP
Dissolving pulp (also called dissolving cellulose) is a bleached wood pulp that has a high cellulose content (> 90%). It is produced chemically from the pulpwood, in a process that has a low yield (30 – 35% of the wood). This pulp has special properties, such as a high level of brightness and uniform molecular-weight distribution.
The minimum requirements for a viscose dissolving pulp for producing bulk products are listed below :
| Alpha-cellulose | >90% |
| Solubility in 5% NaOH | <5% |
| Extractives | <0.3% |
| Ash content | <0.1% |
| Iron | <12ppm |
| SiO2 | <50ppm |
| Calcium | <250ppm |
| Brightness | >90%, ISO |
Manufacture
Dissolving pulp is made from the sulfite process or the kraft process with an acid prehydrolysis step to remove hemicelluloses.
The sulfite process produces pulp with a cellulose content up to 92 percent. It can use ammonium, calcium, magnesium or sodium as a base. The prehydrolysis sulfate process produces pulp with a cellulose content up to 96 %.
Special alkaline purification treatments can yield even higher cellulose levels: up to 96 percent for the sulfite process and up to 98 percent for the sulfate process.
Applications
Dissolving pulp is used in production of regenerated cellulose. The cellulose is dissolved in an organic solvent and processed to regenerate the cellulose fibres in different forms.
The 90-92 % cellulose content sulfite pulps are used mostly to make textiles (like rayon) and cellophane. The 96-% cellulose content sulfate pulps are used to make rayon yarn for industrial products such as tire cord, rayon staple for high-quality fabrics, and various acetate and other specialty products.
As a raw material of cellulose derivatives, dissolving pulp is used in carboxymethyl cellulose (CMC), methyl cellulose (MC), hydroxypropyl cellulose (HPC), hydroxyethyl cellulose (HEC), etc.
Since dissolving pulp is highly refined, it is a product of high whiteness with few impurities making it suitable in specialty paper-related products such as filter paper and vulcanized fibre.
Cellulose powder is dissolving pulp that has undergone acid hydrolysis, been mechanically disintegrated and made into fine powder.
This pulp is used as a filler for urea-formaldehyde resins and melamine resin products.
SEMICHEMICAL PULPING
Semichemical pulping processes are characterised in principle by a chemical treatment preceded by a mechanical refining step for defibring or fibrizing. This general definition also applies to the chemimechanical processes and the high-yield chemical pulping processes such as the sulphite and kraft. It is more suitable to hardwoods. The chemical treatment in semichemical and chemimechanical pulping can be effected with reagents like sodium sulphite, caustic soda, and kraft liquor. The sodium sulphite is usually buffered to near neutrality with sodium carbonate or bicarbonate or kraft green liquor.
Neutral sulphite semichemical process (NSSC)
The most important semichemical process is undoubtedly the neutral sulphite semichemical (NSSC) process . The general advantages of the NSSC process are low requirements with regard to wood quality and species, high yields, relatively low consumption of chemicals at a given residual lignin content, low capital investment and profitable small production units as compared to full chemical pulping. Besides single hardwoods and hardwood mixtures, mixtures of hardwoods and softwoods can also be pulped successfully with the NSSC process. NSSC pulping is also used for non-wood plants and residues because of the generally low lignin contents of these materials and the widely variable conditions offered by the NSSC pulping.
The principal process involves impregnation with a neutral sodium sulphite pulping liquor at about 125 0C for an hour under pressure after a short steaming of the chips at atmospheric pressure, followed by cooking at temperatures between 160 and 190 0C, depending on the cooking time, which may vary between 15 minutes and 8 hours, which again depending on the type of digester used and the desired pulp type and quality. Defibration is carried out by means of single or multi-stage refinement process using disk refiners.
The lignin content of the NSSC pulps is high as compared to those from chemical pulps, and ranges between 10 and 15 per cent. Due to the high lignin and polyoses content the NSSC pulp has low conventional strength properties. The typical NSSC pulp is normally much more rigid and stiff than kraft pulp. Therefore, it is the most typical and suitable fibre material for the production of corrugating medium.
The caustic soda in the soda semichemical process reacts with the lignin-carbohydrate complex to form soluble sodium lignate, and the carbohydrates are solubilized by hydrolysis. However, this lignin reaction occurs only after a major portion of the caustic soda has been consumed in neutralizing the readily available acetyl and methoxyl groups and in hemicellulose dissolution. Therefore, lignin removal is the least in alkaline semichemical pulping process.
Cold-soda semichemical process.
The cold-soda or cold caustic process, which is less important than the NSSC process, involves in principle, the treatment of chips with a sodium hydroxide solution at temperatures generally between 20 and 30 0C and a final refiner defibration. In the cold-soda chemimechanical pulping, the caustic soda attacks the fibre bond mainly by reaction with the acetyl and other acid groups, which are reactive even at room temperature. The most important step in the cold-soda pulping is the impregnation with alkaline liquor to reach a very fast but total penetration of the chips, causing the necessary swelling of fibres and avoiding considerable losses of polyoses. Impregnation times are between 15 and 120 minutes with generally short reaction times of 15-30 minutes in pressurized and continuous systems. The concentrations of NaOH are generally low (0.25–2.5%), but up to 10 per cent in the case of some roller mill impregnation systems. Cold-soda pulping requires little installation capital, and despite the cost of the chemicals, the processing costs are actually lower than in stone grinding, because of reduced energy consumption. In some process modifications the liquor is reused up to 20 times. The cold-soda pulp yield ranges between 85 and 92 per cent, whereby the selectivity of lignin and polyoses dissolution is much lower than NSSC pulping.
The main disadvantage of cold-soda pulps is generally a low brightness level (40-50%), which can be effectively increased by a two-stage peroxide-hypochlorite bleaching. As the cold-soda pulps have properties comparable to NSSC pulps, these are used as unbleached coarse grades for corrugating medium production, and as bleached grades for printing papers and newsprint in combination with groundwood pulp and chemical pulp.
In the kraft semichemical process, the reactions of kraft liquor are similar except thiolignin is formed and dissolved. Because of the buffering nature of the sodium sulphhydrate in the kraft liquor, attack on the hemicelluloses and cellulose is less in the kraft than in the soda process.
In the acid sulphite and bisulphite semichemical pulping, the delignification reaction predominates under acid conditions. As with the NSSC process, the mechanism of delignification probably involves sulphonation of the lignin of the middle lamella in the solid state, followed by hydrolysis to soluble lignosulphonic acid and carbohydrates. The hemicelluloses are less dissolved in these acid procedures than in the others.
In the sulphite chemimechanical pulping, the neutral or acid sodium sulphite solutions dissolve mainly carbohydrates. The relatively high temperatures employed have an important weakening effect on the fibre bond. The material from the chemical stage may be partially disintegrated and defibred through the mechanical action of digester discharging, conveying, or deliquoring. The first action in the defibring–refining machine is probably heating the fibre bond and further weakening it to the point that it will split. Temperature is an important factor in fibrizing semichemically softened fibrous material. The second action in the mechanical state is the actual disintegration of the fibre aggregates and separation into individual fibres. The third action in the fibrizing zone of the machine, which is generally superimposed on the defibring action, is the refining or processing of the individual fibres to prepare them for papermaking. This action, which largely involves fibrillation, softening, and formation of colloidal, mucilage-like surfaces, is for the purpose of stock preparation as with any other type of pulp. The actions occurring during the mechanical part of semichemical pulping may take place in one or more stages or passes. This is determined by a number of factors including the kind of fibrous material and its particle size, the degree and kind of chemical treatment, and the requirements for papermaking.
The semichemical pulps have chemical and strength properties intermediate between groundwood and full chemical pulps. The brightness of these pulps varies from 35 to 55 per cent which can be improved to 80 to 85 per cent by multi-stage bleaching. The semichemical pulps are characterized by their high lignin and hemicellulose (pentosans) contents.
Comparison of three types of chemical pulping process
| Types of process | Kraft or sulfate pulp(alkaline) | Sulfite pulp(acid) | NSSC pulp |
| Cellulosic raw material | Almost any kind of wood,soft or hard | Coniferous; must be of good color and free of certain phenolic compounds. | Mainly Hardwood used some soft wood (small chip size,fiberized) |
| Principal reaction in digester | Hydrolysis of lignins to alcohols and acids;some mercaptans formed | RC:CR’+Ca(HSO3)→ |
(RCHCR’SO3)2CaLignin sulfonation and hemicelluloses hudrolysis lead to formation of acetate and formate.Composition of cooking liquor12.5% solution of NaOH,Na2S and NaCO3.Typical analysis of solids;58.6%NaOH,27.1%Na2S,14.3%NaCO3.Dissolving action due toNaOH and Na2S.NaCO3 inactive and represents the equilibrium residue between lime and NaCO3 in the formation of NaOH.7% by weight SO2,of which 4.5%is combined as sulfurous acid and 2.5% as calcium or Mg(HSO3)2.Cooking 1ton of pulp requires 175 to220kg of SO2 and 55 to 68 kg of MgO.Recent significant trend toward use of Mg(OH)2,NH4OH as base to speed lignin solution.Na2S buffered with NaCO3 ,bicarbonate or kraft green liquor.Concentration of 90-100g/L of Na2S.Cooking liquor does not complete freeing of fibers,but mechanical treatment does.Cooking conditionsTime 2-5h;temp.170-176ᵒC.pressure660-925kpaTime 6-12h;temp.125-160ᵒC or higher;pressure 620-755kpaTime 36-48 min;corrugating grade pulp from hardwoods 12-15min;temp.160-180ᵒC.pressure660-1100kpa.Chemical recoveryMost of process is devoted to be recovery of cooking chemicals,with incidental recovery of heat through burning organic matter dissolved in liquor from wood;chemical losses from system are replenished with salt cake,NaSO4.SO2 relief gas recovered;magnesium liquor recovered and reused after digestion and pulp washing.Characterized by high yield 65-85%;pulping losses 35-15% of wood components.Special recovery methods and by-product utilization.Materials of constructionDigester,pipelines,pumps and tanks can be made of mild steel or,preferablyof stainless steel.Acid liquor requires digester liling of acid proof brick;fittings of chrome-nickel steels(type 316) ,lead,and bronze.Serious corrosion problems encountered in digesters and handling equipment;stainless steel protection needed.Pulp characteristisBrown color;difficult to bleach;strong fibres;resistant to mechanical refining.Dull white color;easily bleached;fibers weaker than kraft.Stiff,dense paper of low opacity;fiber approach chemical pulps in strength.Typical paper productsStrong brown bag and wrapping,multiwall bags,gumming paper,building paper,strong white paper from bleached kraft,paperboard such as used for cartons,containers,milk bottles .and corrugated board.White grades;book paper.bread wrap,fruit tissue,sanitary tissue.Unbleached;large percentage for corrugated board,also newsprint,speciality board.Bleached;writing and bond papers,offset, mimeo,tissue and toweling.
SOLVENT PULPING (ORGANOSOLV)
Organosolv is a pulping technique that uses an organic solvent to solubilise lignin and hemicellulose. It has been considered in the context of both pulp and paper manufacture and biorefining for subsequent conversion of cellulose to fuel ethanol. The process was invented by Theodore Kleinert as an environmentally benign alternative to kraft pulping. Organosolv has several advantages when compared to other popular methods such as kraft or sulfite pulping. In particular, the ability to obtain relatively high quality lignin adds value to a process stream otherwise considered as waste. Organosolv solvents are easily recovered by distillation leading to less water pollution and elimination of the odour usually associated with kraft pulping.
Organosolv pulping involves contacting a lignocellulosic feedstock such as chipped wood with an aqueous organic solvent at temperatures ranging from 140-220°C. This causes lignin to break down by hydrolytic cleavage of alpha aryl-ether links into fragments that are soluble in the solvent system. Solvents used include acetone, methanol, ethanol, butanol, ethylene glycol, formic acid and acetic acid. The concentration of solvent in water ranges from 40-80%. Higher boiling solvents have the advantage of a lower process pressure. This is weighed against the more difficult solvent recovery by distillation Ethanol has been suggested as the preferred solvent due to cost and easy recovery. Although butanol is shown to remove more lignin than other solvents and solvent recovery is simplified due to immiscibility in water, its high cost limits its use.
Organosolv for pulp production
Numerous authors report that pulping with ethanol-water solutions gives a lignin free pulp yield 4-4.5% higher than that of kraft pulp. The commonly used solvents acetone and ethanol have been examined with respect to pulp properties. The pulping of wheat straw with 40% mixtures of acetone or ethanol with water requires 60 minutes at 180°C to give good pulp properties. Organic solvents are almost always used as a mixture with water for process considerations such as reducing the vapour pressure and lowering the pH in order to also solubilise hemicellulose.
Organosolv for fuel ethanol production
Recently, due to the popularity of 2nd generation biofuels, the organosolv process has been considered in the context of bioethanol production. Cellulose from the organosolv process is amenable to enzymatic hydrolysis into glucose followed by fermentation to dilute ethanol. The organosolv fractionation of mountain beetle killed lodgepole pine has yielded 97% conversion to glucose. Panetal. recovered 79% of the lignin using conditions of 170°C, 1.1% w/w H2SO4, 65% v/v ethanol for 60 minutes.
Lignin recovery
The recoverey of lignin from ethylene glycol organosolv pulping can be effected by 3 times dilution with acidified water. The lignin precipitates and forms spherical aggregates ranging from 0.5-2.5µm. Filtration, while time consuming, is then most effective while the mixture is hot (>100°C). Recovery can be achieved by filtration or centrifugation. Due to the hydrophobic nature of organosolv lignin, flotation of organosolv is effective without the use of collecting and precipitating agents that are required for flotation of kraft lignin lignin has proven especially easy due to the hydrophobic nature of organosolv lignin .
Processes
Organocell uses two stage organosolv with roughly 50% methanol solutions. Sodium hydroxide is added in the second stage at a loading of 30% w/w of the dry wood. The lignin from the second stage is isolated by adding phosphoric acid until a pH of 4.0 is reached. The Alcohol Pulping and Recovery (APR) process treats wood in 3 stages, each using increasingly cleaner solvent. The important process parameters are extraction time, temperature, solvent composition and pH. Pilot plant operation has shown that ethanol pulping produces pulp superior to sulphite pulp at a lower cost. Lignin and hemicellulose are recovered in high yields. In 1987 the APR process was renamed the Alcell process. The process uses aqueous ethanol solutions (40-60% v/v) to delignify wood at temperatures from 180-210°C and 2-3.5MPa. Solvent is recovered with flash evaporation, vapour condensation and vacuum stripping. A demonstration organosolv pulp mill has operated in Mirimichi, New Brunswick, Canada from 1989-1996 using the Alcell process. Repap owned the IP to the process when taken over by hedge funds in 1997. The pilot plant boasted superior environmental performance, excellent bleached pulp, an economically attractive scale of 300tons/day and commercially attractive by-products. It is said that the technology can be used to exploit small regions of hardwood resource that could not support a modern sized kraft mill.
RICE PAPER FROM RICE PULP
Rice paper is made from the pulp of the rice plant. Rice paper has an unusual texture and is typically somewhat translucent. This is a traditional Asian paper popular for use in a variety of crafting-project.Rice paper is readily available in crafting stores, actually classified as “faux” rice paper because it is made from mulberry leaf pulp.
Making Rice Straw Pulp
· Rice straw is typically available at local farm stores and feed stores and is quite inexpensive. Discard all parts of the stems except for the straight parts of the stem between the nodes. Cut the rice straw into 1-inch sections and remove any leaves.
· Soak the rice straw in a bucket of cold water for at least one week, preferably 10 days. Monitor the color of the water in the bucket. When it turns brown, replace it with fresh water. This should be done several times during the soaking process.Rinse the rice straw completely at the end of the soaking process.
- Use 15 quarts of water and 26 ounces of wood ash for each pound of dry rice straw. Boil the wood ash for approximately 30 minutes and then allow it to sit undisturbed for 24 hours.
- Wear thick rubber gloves to protect hands and wear clothing that covers arms and legs. This wood ash solution is very caustic.After 24 hours, strain to remove the wood ash from the water. It is important not to allow the water to contain any wood ash particles. Line the strainer with cheese cloth if necessary to prevent wood ash from filtering through.
- Cook the soaked rice straw in the wood ash water in a large stock pot.Boil for at least three hours, but monitor the boiling process. The wood ash water will be breaking down the rice straw fibers as it boils. The rice straw has broken down sufficiently when it crumbles easily. Be careful not to overcook the rice straw because this will produce paper that is not strong.After the boiling process, rinse the rice straw in clean, cold water.
- Drain the cooked rice straw and spread it out on a tarp. Pound the rice straw with a baseball bat. Excessive force is not necessary. This process will loosen the rice straw fibers from the rest of the rice straw. When the rice straw fibers are spread out on the tarp, push the fibers back together and continue beating with the bat. The goal is to beat the rice straw fibers into a fine pulp.
Future fibres for pulp mills in Bangladesh
Bangladesh are facing acute shortage of fiber for pulp and paper industry. On the other hand, the demand of paper and paper products is increasing day by day. Nonwood or agro-based fibers are potential sources for pulp production. However, these fibers have tremendous variations in chemical and physical properties compared to wood. This study presents the availability of nonwood raw materials for pulp production in Bangladesh and focused its quality. It is also discussed the problems related to nonwood pulping. Bangladesh have huge amount of unused jute fiber, which is the best source for pulp production in Bangladesh. Other agricultural wastes like cotton stalks, corn stalks, Dhaincha, Straw etc. may be used for pulp production. Jute pulp may be used as reinforcing agent with other nonwood pulps for better quality paper. Big pulp mills could be established based on jute and a few small pulp mills may be set up based on agricultural wastes. An integrated pulp mill may also be established depending on different nonwood raw materials.
The basic raw materials for pulp and paper industries come from forest. Currently 90% of chemical and mechanical pulps are produced from wood. The global consumption of paper and board increased to 314.4 millions ton in 1999 from 231.6 million ton in 1989 (Anon 1990; 2000). This increase is expected to rise further with increasing world population, literacy rate, and quality of life. The continued high growth in paper consumption will lead to increase demand of fiber, creating additional pressure on the world diminishing forest resources. To maintain the growth of paper industry, thereby increasing supply of raw materials, government as well as executive of industry have to take a policy of reforestation, plantation management, recycling and development of alternative fibrous raw materials, such as annual plant, agricultural wastes, which are commonly called nonwood.
In Bangladesh paper industry is using bamboo and mixed hardwood. Both of these raw materials come from forest. Presently about 38,000 acrors of forest is being used for pulp and paper industry. Tribal peoples live in the forest region of Bangladesh. Traditionally, they use land for jhoom cultivation. Therefore, huge amount of forest is destroyed during jhoom cultivation. Hence allocated forestland for paper industry could not meet the demand. On the other hand the gap between demand and supply of pulp and paper in Bangladesh is increasing day by day. Therefore, more pulp and paper industry is needed which demanding more fibrous raw materials. This article describes the possibility of utilization of nonwood raw materials for pulp production.
NONWOOD FIBERS
In general, fibers can be classified into three categories wood, nonwood and nonplant. The term `nonwood’ was to distinguished from the two main sources of wood fiber hardwoods and softwoods. Nonwood or ago-based fibers are derived from selected tissue of various mono- or dicotyledonous plants and are categorized botanically as grass, bast, leaf or fruit fibers.
Some nonwood fibers are classified by means of production; fibers such as sugar cane bagasse, wheat straw and corn stalks are by products. Other nonwood fibers are grouped as “fiber plants” plants with high cellulose content that are cultivated for the sake of their fibers such as jute, kenaf, flax etc.
GLOBAL AVAILABILITY OF NONWOOD FIBER
In commercial development, there must be a long term guaranteed supply of resources. In order to insure a continue fiber supply, management of the agricultural producing land should be under a proactive system of land management whose goal is both sustainable agriculture and the promotion of healthy ecosystems.
Worldwide, nonwood pulping capacity was 23 million ton/yr in 1997, which represented 10.9% of total paper pulp capacity. By 1998, it was reached to 24.1 M ton/yr (11.2%). Since 1975, nonwood pulping capacity has grown at more than double the rate of wood pulping capacity, with the period 1993-98 averaging 3.4% pa (as compared with 1.2% pa for wood pulping). Most nonwood pulp is used in integrated mills, but some pulps and raw material are imported into developed countries for the manufacture of specialty grade papers and environmentally friendly paper grades. Some long fiber length nonwood pulps are used in nonwovens (eg: cotton).
Table- shows the estimated annual availability of nonwood fibrous raw materials worldwide – and thus the potential material for further expansion. Corn stalks contribute the highest amount of nonwood fiber. Among the straw fibers wheat straw contribute largest amount followed by rice straw.
CHEMICAL AND PHYSICAL PROPERTIES OF NONWOOD
The data of chemical and morphological characteristics of nonwood shows wide variation from one raw material to others (Table 3). Nonwood raw materials contain lower lignin compared to wood. Its fiber length is similar to hardwood. But fiber length of jute and bamboo is similar to softwood. Ash content is higher as compared to wood, which creates difficulties during recovery. Rice straw contains highest amount of ash.
PULP PROPERTIES OF NONWOOD PULPS
On a worldwide basis, the future use of nonwood fibers on a large scale is indeed a reality. For a medium- and long-term future, greater use of these raw materials in the world will become a reality as well. About 11% of world pulp production are made from nonwood fibrous raw materials, some of which are agricultural raw materials. Most nonwood pulp is produced in China and India. These two countries account 80% of the total nonwood pulp production. Bangladesh have potential nonwood fibrous sources for pulp production. Pulping properties of a few important nonwoods of Bangladesh is given below
Jute
Jute is abundantly grown in Bangladesh. Jute has a long historical role in the socioeconomic development of Bangladesh. Once jute was known as golden fiber of Bangladesh. The export of jute and related products accounts for a significant portion of total export. In addition, it provides considerable employment opportunities to the country’s work force. Traditionally jute was used in backing, sacking, gunny bag, hasein etc. In recent years, jute has faced stiff competition from synthetics. Therefore, traditional uses of jute have declined. As a result, its demand in local and overseas markets has been shrunk. This has caused jute prices drop and jute growers are the ultimate victims. Therefore, diversified usage is needed to regain the lost glory of jute. The chemical and morphological characteristics of jute favor it as pulping raw material.In the northern region of Bangladesh, about 0.2 million ton of jute remain unused in every year due to its low quality in traditional uses. Therefore, farmers don’t get proper price. But it produce a good quality pulp, pulp yield is above 60% with kappa number less than 20. The papermaking properties are very high like softwood pulp.The SR number of unbeaten pulp is below 15, which are acceptable for subsequent processing. Utilization of jute in pulp production creates a new horizon for farmers.
If whole jute plant is used in pulping, pulp yield is about 45-50% with kappa number about 20-25. Whole jute plant consists of stick (woody portion) and bark (fiber) in the ratio of 2.5:1. Stick contains high lignin and has short fiber length, hence pulp produced from whole jute plant shows higher tensile but low tear strength as compared to jute fiber pulp. The SR number is above 15, so whole jute plant pulp need special design equipment for pulp washing.
Straw
Straw can be an important source of raw materials for the production of paper. Cereal straw, in particular wheat straw is a major source of pulp for paper production in China and other Asian countries. The high silica content of rice straw (9-14%) however prohibits the economic use of rice straw for this purpose. The silica will cause problems in the recovery of chemicals used in the pulping process. For rice straw, there is currently no commercially available solution for this problem. Other problems with the use of straw for pulp are the higher water retention capacity of straw, the lower yield per ton of raw material compared to wood (straw yields 45% of pulp whereas wood yields 55% pulp), and the low bulk density of straw. A little study has been made on straw pulping in Bangladesh. Bangladesh produce huge amount of rice straw and wheat straw. Therefore, more study should be done straw pulping. A method should be developed on the desilication of rice straw.
Cotton stalks
After cotton cultivation, stalks are remained in the field. Now it is being used as domestic fuel in the rural area in a limited extent and rest is left in the field. Disposal of the stalks increases production cost of the farmer. Therefore, it is burnt in the field, which is not environmental friendly. Utilization of this agricultural waste reduces the production cost of cotton. It is a wood like nonwood raw material. The chemical and morphological properties of cotton stalks are comparable to hardwoods. The pulp yield is about 40-45% with kappa number 30-35 . The SR number of unbeaten cotton stalks pulp is about 12-15. The tensile strength of cotton stalks pulp is very high but tear index is very low. Blending cotton stalks pulp with jute pulp could increase tear index.The bleachability of cotton stalks pulps is very good in ECF bleaching.
Corn stalks
The chemical and morphological characteristics of corn stalks are comparable to hardwood species. It is easier to delignify than wood. Corn stalks require lower temperature for pulping. The pulp yield is about 50% at kappa number 20. The papermaking properties are very good except teat strength. Blending of corn stalks pulp with jute pulp increased tear strength. Due to high fine content water retention is very high. Therefore, pulp-processing equipment should be similar to straw pulp mill.
Bagasse
One pulp mill in Bangladesh is based on bagasse. Bagasse is a by-products of sugar mills. Sugar mills in Bangladesh use bagasse as fuel for steam generation. Therefore, sugar mills consume almost all bagasse. Recently, Bangladesh government connects gas pipeline in the northern region, where almost sugar mills are situated. If sugar mills use this gas for steam generation then bagasse can be used for pulp production. Pith is the main problem for bagasse pulping. It creates problem during pulp washing, clogging in machine wire etc. Therefore, pith must be removed before pulping and also fiber treatment is needed.
Kash (Saccharum spontaneum)
Saccharum spontaneum is produced the wet and sandy land. It is similar to bagasse. In Bangladesh it is called Kash and in India it is Kan grass. Now it is used as fencing in rural area to a limited extent. About 20 thousands metric tons of S. spontaneum is available in Bangladesh. Pulp yield from Saccharum spontaneum is very high and kappa number is very low. The initial brightness (about 50%) of S. spontaneum pulp is suitable for newsprint. The papermaking properties are comparable to tropical hardwood.
Dhanicha
Dhanicah is grown in sandy region of Bangladesh. It protects land from erosion. It also fixes nitrogen to the soil. Therefore, it is most useful annual plant for our agricultural system. Now it has no industrial uses but it is used as domestic fuel in the rural area. Chemical and morphological properties favor it as pulping raw material. Pulp yield and kappa number are similar to hardwood. The paper making properties of dhanicha are also similar to hardwood.
From this investigation it may be concluded that:
- Jute is the best source for pulping in Bangladesh.
- Cotton stalks, Corn stalk, Dhanicha, Straw etc. may be used as pulping raw materials in Bangladesh.
- Blending of jute pulp with other nonwood pulps may produce better quality of pulp.
- An integrated pulping system may be adapted for nonwood pulping.
- Inventory of nonwood raw materials in Bangladesh is needed.
Non-conventional pulping processes
The non-conventional pulping processes include well-known pulping principles, which are, however, only rarely applied commercially. Their industrial application is often limited by high costs of chemicals and special equipment requirements. However, they posses the advantage of more environmental friendliness techniques. Pulping via sodium-xylene sulphonate, aqueous ethanol, ketone-ammonia mixture, ethanol amine, peracetic acid, oxygen-alkali, etc., are some of the efforts to develop pollution free pulping processes. Among the various processes, some commonly referred methods are described below :
Ethanol-water pulping
Aqueous ethanol is a powerful delignifying agent. Retention of lignin is strongly dependent on pH of the cooking liquor. After distilling off the alcohol from ethanol-water black liquor, a major fraction of the solubilized lignin separates as a quasi-molten phase.
Hydrotropic pulping
Certain substances which are only slightly soluble in water become more soluble in the presence of certain salts known as hydrotropic salts. These are salts of organic acids which have a large organic group and are themselves soluble in water. It has been shown that a near-saturated aqueous solution of sodium xylen sulphonate at approximately 700C, will dissolve large quantities of lignin. These salts appear to act as catalysts. As they are not consumed, they can be recovered unchanged for reuse. Also they hydrolyse wood or straw at a much faster rate than the chemicals used in other processes of pulp making. In pulping with sodium xylene sulphonate, cooking could be accomplished in shorter time and at lower temperature than with other standard processes. For most woods the composition of the hydrotropic cooking solution is approximately one third salt and two thirds water; the solution is adjusted to a slightly acid pH (3.5). A typical cook, for paper bags takes 2-3 hrs. at 145 0C and 4 bar pressure. At the end of the cook, the solution is filtered from the pulp and reused half a dozen times for other cooks. The solution is then diluted with warm water (40- 600C) to a salt concentration of 8-10 per cent for precipitating the lignin. The lignin is removed by filtration and the solution is evaporated until the concentration of sodium xylenesulphonate is nearly 30 per cent; at this stage the solution can once again be used for cooking. The solution is nonscaling and noncorrosive and free from objectionable odour. The pulp is pressed to a consistency of about 40 per cent, washed free of the remaining hydrotropic salt. The properties of the pulp fall between those of an acid sulphite and an alkaline kraft pulp. The yield is 10 per cent higher and gives a high white colour in the usual bleaching processes.
Nitric acid pulping
The nitric acid pulping process consists of a two- stage pulping operation. In the first stage nitric acid is used to remove the lignin and, to a lesser degree, the pentosan and other non-cellulosic materials without damaging the cellulose fibre. The chemical reactions involved are nitration, oxidation and hydrolysis. The second stage consists of an alkaline extraction of the nitrolignin residues encrusting the cellulose fibre. I to 4 per cent sodium or ammonium hydroxide is normally used to dissolve and disperse these residues.
Although high-yield pulps (45- 52%) displaying good physical strength properties intermediate between kraft and sulphite pulps have been obtained under a wide range of pulping conditions, the high cost of the acid, which must be used in relatively large concentrations (52%) and lack of an efficient recovery system caused a bottle-neck for commercial adoption of this process on a wide scale.
Modified method of nitric acid pulping of bagasse
Pulping of bagasse by a rapid and mild nitric acid process was successfully carried out to produce different grades of pulp. Nitric chemimechanical bagasse pulp was produced in a high yield of 91% on pulping depithed bagasse with 4% HNO3for a period of 30 minutes at 80 degrees C, followed by alkali pulping with 2% NaOH at 95 degrees C for 30 minutes. The pulp had a satisfactory strength and high opacity. On increasing the strengths of nitric acid to 7% and alkali to 7% a nitric semichemical pulp of 65% yield was obtained. The pulp had a superior strength and high opacity. The pulp was easily bleached to 71% general electric brightness (GE) with the chlorination-alkali-extraction-hypochlorite (CEH) sequence. With 15% HNO3and 8% NaOH, nitric chemical bagasse pulp was produced. The pulp was easily bleached to a high brightness of 82% GE with one stage hypochlorite. The pulp had a higher strength than kraft bagasse pulp. A satisfactory newsprint paper was produced on an experimental paper machine with a furnish composed of 80% bleached nitric semichemical bagasse pulp, 10% bleached softwood pulp and 10% clay.
Peracetic acid pulping
Lignin degradative oxidation is used in the pulping process with peracetic acid. This reagent selectively delignifies and gives pulp in high yield but requires large amounts of expensive chemicals and therefore not economical.
Sulphur free chemical pulping processes
Environment protection concerns led to the development of sulphur free chemical processes as alternatives to sulphite and kraft pulping. Non-sulphur alkaline pulping covers delignification procedures such as, two-stage soda-oxygen process, single-stage oxygen process, and the alkaline-AQ-peroxide process.
The two-stage soda-oxygen pulping process involves a high-yield soda cook, followed by a mechanical defibration and a concluding oxygen bleaching in alkaline medium. The cooking stage has the greatest influence on final pulp yields and properties. The yield after the cooking stage should be in the range of 60-65 per cent to reach high final yields after the bleaching procedure, and to achieve optimal refining conditions with regard to energy input and fibre protection. The yields of bleached pulps are comparable to kraft pulp yields, while the strength properties are somewhat lower. A practical aspect of this process is the fact that it can run in established kraft pulp digestion and recovery equipment with an added refining section and an oxygen bleaching plant.
In the single-stage oxygen pulping process, the delignification is carried out in slightly alkaline solutions (pH 7-9) of sodium hydroxide, sodium carbonate or hydrogen carbonate (also in the presence of hydrogen peroxide) at 140-150 0C and high oxygen pressures in the range of 20-40 bar. The pressure cooking is carried out at low consistency to maintain the temperature level. To avoid insufficient oxygen penetration into the wood very thin chips (< 1.5 mm) must be used. The pulps are generally obtained in high yields and good brightness, but the strength properties are usually not comparable to those of kraft pulps. Strength improvements can be obtained by sodium hydrogen carbonate pre-cooking, increasing the carbon dioxide content in the gas phase, and by addition of potassium iodide or other metal compounds as cellulose protectors.
In the alkaline–AQ-peroxide process, delignification is carried out in two stages with a refining step in between. The first stage is a high-yield soda-AQ cook down to kappa numbers of 50-60. The second delignification step is performed as a medium to high consistency (>10%) hydrogen peroxide bleaching (<0.5% H2O2 based on wood), reducing the kappa number down to about 30. The obvious advantages of this process are: 2-5 per cent higher yields than in kraft pulping, better mechanical characteristics and higher brightness as compared to kraft and soda-AQ pulps, low AQ amounts (<0.1%), elimination of the air pollution problems of kraft pulping, and easy and low-cost substitution within the existing kraft mills by installation of a refiner section and a hydrogen peroxide bleaching tower.
Apart from the non-sulphur alkaline pulping processes, the organosolv pulping is a sulphur free non-alkaline process employing 1:1 mixture of alcohol (ethanol or methanol or butanol) and water or phenol and water as the cooking medium. Organosolv pulping seems to be a viable future pulping alternative because of the relatively low capital investment required for a new mill, the absence of pollution problems, and the advantage of obtaining polyoses and lignin easily and largely unchanged for further high-value utilization.
Bio-pulping
As microorganisms or the enzymes produced by them can break down lingo-cellulosic materials, preferential degradation of lignin by soft-rot fungi can be exploited for achieving at least partial delignification. Considerable amounts of the complex lignin molecule can be degraded or altered to bring about savings in energy inputs during pulping. The cleaving of carbon-carbon and carbon-oxygen linkages by the biological process renders the lignin molecule more accessible to subsequent action of the cook liquor. While the industrial physico-mechanical process operated under high pressure and temperature, the biological conversion process operates under much milder conditions thus resulting in energy savings.
A systematic screening of a number of white rot fungi which could act preferentially to degrade middle lamella lignin leading to defibration, led to the identification of an isolate of Schizophyllum commune. Currently the time required after treatment is in the order of days and can be reduced with the identification of right strains and other treatment conditions. The treatment can be done either during transport or storage in the yard.
ECONOMIC IMPORTANCE
The manufacture of pulp, paper and paper products ranks among the world’s largest industries. Mills are found in more than 100 countries in every region of the world, and directly employ more than 3.5 million people. The major pulp and paper producing nations include the United States, Canada, Japan, China, Finland, Sweden, Germany, Brazil and France (each produced more than 10 million tonnes in 1994.
Table – Employment and production in pulp, paper, and paperboard operations in 1994, selected countries.
| Country * | Number employed in industry | Pulp | Paper and paperboard | ||
| Number of mills | Production (1,000 tonnes) | Number of mills | Production (1,000 tonnes) | ||
| Austria | 10,000 | 11 | 1,595 | 28 | 3,603 |
| Bangladesh | 15,000 | 7 | 84 | 17 | 160 |
| Brazil | 70,000 | 35 | 6,106 | 182 | 5,698 |
| Canada | 64,000 | 39 | 24,547 | 117 | 18,316 |
| China | 1,500,000 | 8,000 | 17,054 | 10,000 | 21,354 |
| Czech Republic | 18,000 | 9 | 516 | 32 | 662 |
| Finland | 37,000 | 43 | 9,962 | 44 | 10,910 |
| Former USSR** | 178,000 | 50 | 3,313 | 161 | 4,826 |
| France | 48,000 | 20 | 2,787 | 146 | 8,678 |
| Germany | 48,000 | 19 | 1,934 | 222 | 14,458 |
| India | 300,000 | 245 | 1,400 | 380 | 2,300 |
| Italy | 26,000 | 19 | 535 | 295 | 6,689 |
| Japan | 55,000 | 49 | 10,579 | 442 | 28,527 |
| Korea, Republic of | 60,000 | 5 | 531 | 136 | 6,345 |
| Mexico | 26,000 | 10 | 276 | 59 | 2,860 |
| Pakistan | 65,000 | 2 | 138 | 68 | 235 |
| Poland** | 46,000 | 5 | 893 | 27 | 1,343 |
| Romania | 25,000 | 17 | 202 | 15 | 288 |
| Slovakia | 14,000 | 3 | 304 | 6 | 422 |
| South Africa | 19,000 | 9 | 2,165 | 20 | 1,684 |
| Spain | 20,180 | 21 | 626 | 141 | 5,528 |
| Sweden | 32,000 | 49 | 10,867 | 50 | 9,354 |
| Taiwan | 18,000 | 2 | 326 | 156 | 4,199 |
| Thailand | 12,000 | 3 | 240 | 45 | 1,664 |
| Turkey | 12,000 | 11 | 416 | 34 | 1,102 |
| United Kingdom | 25,000 | 5 | 626 | 99 | 5,528 |
| United States | 230,000 | 190 | 58,724 | 534 | 80,656 |
| Total worldwide | approx 3,500,000 | 9,100 | 171,479 | 14,260 | 268,551 |
* Countries included if more than 10,000 people were employed in the industry.
** Data for 1989/90 (ILO 1992).
Source: Data for table adapted from PPI 1995.
Every country is a consumer. Worldwide production of pulp, paper and paperboard was about 400 million tonnes in 1993. Despite predictions of decreased paper use in the face of the electronic age, there has been a fairly steady 2.5% annual rate of growth in production since 1980. In addition to its economic benefits, the consumption of paper has cultural value resulting from its function in the recording and dissemination of information. Because of this, pulp and paper consumption rates have been used as an indicator of a nation’s socioeconomic development.
Conclusion
The main applications for pulp are paper and board production. Chemical pulps are used for making nanocellulose. Speciality pulp grades have many other applications. Dissolving pulp is used in making regenerated cellulose that is used textile and cellophane produciton. It is also used to make cellulose derivatives. Fluff pulp is used in diapers, feminine hygiene products and nonwovens. The furnish of pulps used depends on the quality on the finished paper. Important quality paprameters are wood furnish, brightness, viscosity, extractives, dirt count and strength.
Chemical pulping methods produce high-quality papers as the chemical cooking dissolves most of the lignin and hemicelluloses present in the wood, resulting in better separation of the cellulose fibers.The sulfite process cooks wood chips in sulfurous acid combined with limestone to produce calcium bisulfite. The combination of sulfurous acid and calcium bisulfite dissolves the lignin in the wood and liberates the cellulose fibers. Sulfite pulp is soft and flexible, is moderately strong, and is used to supplement mechanical pulps (most typically in newsprint). Problems with the process (including limitations on the types of trees for which it is suitable, strict pollution laws, and the inability to recover some of the chemicals ejected by the system) have resulted in new chemicals being used in the process, and the wholesale adoption of new processes.
The sulfate process is now the most widely used chemical pulping system. It evolved from the soda processes developed in the nineteenth century, which used strong bases (alkaline solutions) such as lye to digest wood. Pulpers began adding sodium sulfate to the soda process, and a significantly stronger pulp was produced. The advantages of kraft pulping include not only increased pulp strength, but also a better heat- and chemical-recovery system which reduces processing costs, its effectiveness in digesting nearly every known species of tree, and the insertion in the process of bleaching processes which increase pulp brightness. The pulp, as the name “kraft” indicates, is also much stronger than pulp produced via other methods and the paper generated from the process runs well on high-speed presses.
To increase pulp whiteness and brightness (unbleached kraft pulp is usually a dark brown color), and to remove residual lignin, chemical pulps are bleached. It is at this point that additional non-fibrous materials called fillers are added to the pulp—a process called loading—and the resulting furnish—the mixture of pulp and fillers—is ready to begin the refining process.
It is also a process that can be regarded as highly efficient due to the possibility of chemical recovery. Chemical recovery is part of the process in which the chemicals used to treat the wood are reused for another purpose. In some cases, this used liquid, which is often referred to as liquor, is used to produce other chemicals. In other cases, the chemicals are used to create energy.
So,although chemical pulp to produce paper is more expensive than using mechanical pulp or recovered paper, it has better strength and brightness properties.