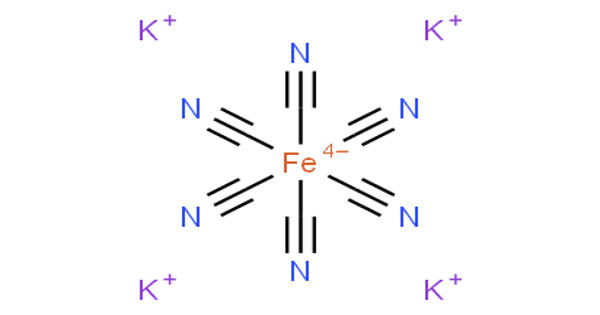
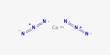
Potassium ferrocyanide is the inorganic compound with the formula K4[Fe(CN)6]·3H2O. It is also known as yellow potash prussiate, a yellow crystal. It is the potassium salt of the coordination complex [Fe(CN)6]4-. This salt forms lemon-yellow monoclinic crystals. It was made with wool or horn clippings stirring hot potassium carbonate with an iron rod.
Potassium ferrocyanide was used for certain iron processes as a developer and as an additive for developers of alkaline pyro.
Properties
- Density: 1.85 g/cm³
- Molecular Weight/ Molar Mass: 368.35 g/mol
- Boiling Point: 400 °C
- Melting Point: 300 °C
- Appearance: Light yellow, crystalline granules
- Solubility: Insoluble in ethanol, ether
Chemical reactions
Treatment of potassium ferrocyanide with nitric acid gives H2[Fe(NO)(CN)2]. After neutralization of this intermediate with sodium carbonate, red crystals of sodium nitroprusside can be selectively crystallized.
Upon treatment with chlorine gas, potassium ferrocyanide converts to potassium ferricyanide:
2 K4[Fe(CN)6] + Cl2 → 2 K3[Fe(CN)6] + 2 KCl
This reaction can be used to remove potassium ferrocyanide from a solution.

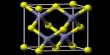
Structure
Like other metal cyanides, solid potassium ferrocyanide, both as the hydrate and anhydrous salts, has a complicated polymeric structure. The polymer consists of octahedral [Fe(CN)6]4- centers crosslinked with K+ ions that are bound to the CN ligands. The K+—NC linkages break when the solid is dissolved in water.
Applications
- Potassium ferrocyanide finds many niche applications in industry. It and the related sodium salt are widely used as anticaking agents for both road salt and table salt.
- It is used in the tempering of steel and in process engraving. It is employed in the manufacture of pigments and as a chemical reagent.
- It is used in the production of wine and citric acid.
- It is used in the manufacture of potassium cyanide, which is used extensively in gold mining.
- It can also be used in animal feed.
- In the laboratory, It is used to determine the concentration of potassium permanganate, a compound often used in titrations based on redox reactions.
- It can be used as a fertilizer for plants.
Toxicity
Potassium ferrocyanide is not toxic to humans since it is not decomposed to cyanide in the body. It is nontoxic, and is not decomposed to cyanide in the body. The toxicity in rats is low, with lethal dose (LD50) at 6400 mg/kg. However, if mixed with strong acids, this compound can be potentially fatal.
Information Source: