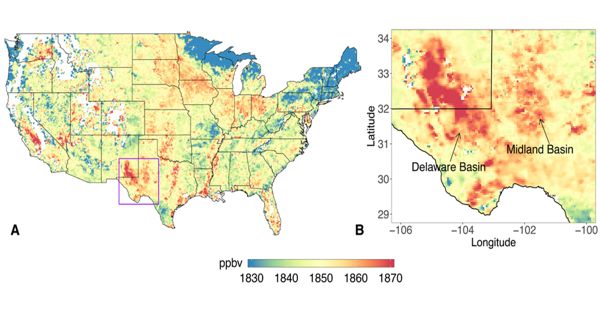
Palladium is a precious gray-white metal, palladium is extremely ductile and easily worked. It is a chemical element with the symbol Pd and atomic number 46. It is a lustrous silver-white metal. It is more reactive than the other platinum metals. For example, it is attacked more readily by acids than any of the other platinum metals.
It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. It was discovered in London. He examined the residues left from platinum after dissolving it in aqua regia, a concentrated solution of hydrochloric and nitric acids. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired by her when she slew Pallas. He then isolated palladium in a series of chemical reactions, finally heating palladium cyanide to extract palladium metal.
Palladium is a chemical element with the symbol Pd and atomic number 46. Classified as a transition metal, it is a solid at room temperature.
Properties
It is a rare, lustrous, silvery-white metal. It is one of the six platinum group metals consisting of platinum, palladium, rhodium, osmium, iridium, and ruthenium.
- Atomic number: 46
- Atomic mass: 106.42 g.mol-1
- Electronegativity according to Pauling: 2.2
- Density: 11.9 g.cm-3 at 20°C
- Melting point: 1560°C
- Boiling point: 2927 °C
- Isotopes: 9
- Vanderwaals radius: 0.065 nm (+2)
- Ionic radius: 0.137 nm
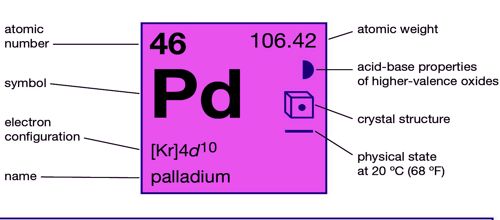
Palladium, platinum, rhodium, ruthenium, iridium, and osmium form a group of elements referred to as the platinum group metals (PGMs). It has a face-centered cubic crystalline structure, at ordinary temperatures, it is strongly resistant to corrosion in air and to the action of acids. They have similar chemical properties, but palladium has the lowest melting point and is the least dense of them.
Occurrences
Palladium has been found uncombined in nature, in Brazil, but most are found in sulfide minerals such as braggite. Russia and South Africa produce about 93 percent of the palladium mined in the world. It is also found in Canada and the United States. It is extracted commercially as a by-product of nickel refining. It is also extracted as a by-product of copper and zinc refining.
More than half the supply of palladium and its congener platinum is used in catalytic converters, which convert as much as 90% of the harmful gases in automobile exhaust (hydrocarbons, carbon monoxide, and nitrogen dioxide) into less noxious substances (nitrogen, carbon dioxide, and water vapor).
Used
Palladium is used extensively in jewelry-making in certain alloys called “white gold.” It is also used in electronics, dentistry, medicine, hydrogen purification, chemical applications, groundwater treatment, and jewelry. It is used in watch bearings, springs, and balance wheels and also for mirrors in scientific instruments. Palladium is a key component of fuel cells, which react hydrogen with oxygen to produce electricity, heat, and water. Palladium salts are used in electroplating.
Information Source: