The most common non-metallic component of manganese is manganese dioxide, used primarily as a depolarizer in dry-cell batteries. It is a black-brown solid with the formula MnO2 that occurs naturally. It occurs naturally as the pyrolusite mineral, which is the main manganese ore and a part of the nodules of manganese. Pyrolusite, which was regarded as a pigment by the ancients, is the primary ore of manganese dioxide. Impure manganese can be produced by carbon-lowering manganese dioxide. It is the only major quadripositive manganese compound. For dry-cell batteries, such as the alkaline battery and the zinc-carbon battery, the primary application of MnO2. As a pigment and a precursor to other manganese compounds, such as KMnO4, MnO2 is also used. For example, it is used as a reagent in organic synthesis to oxidize allylic alcohols.
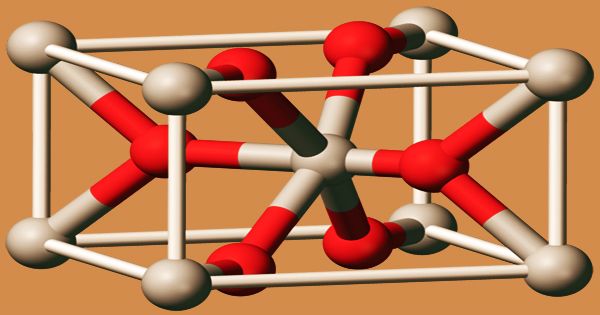
Crystal structure of manganese dioxide
Manganese dioxide takes in electrons through a redox reaction in both standard alkaline and rechargeable battery cells, becoming Manganese III oxide. In lithium-ion technologies, it also has applications. MnO2 is α polymorph that can integrate a number of atoms (as well as water molecules) between the manganese oxide octahedra in the “tunnels” or “channels” Iron-black to dark steel-gray or mineral-bluish; stripes of black or bluish-black; lustre of shiny or dull. Hydrochloric acid solubility. In the presence of oxygen, manganese dioxide reacts with potassium hydroxide and forms potassium permanganate.
4MnO2 + 4KOH + 3O2 → 4KMnO4 + 2H2O
Manganese dioxide reacts with aluminium, forming water and aluminium oxide. As follows, the chemical equation is:
MnO2 + Al → Al2O3 + Mn
As well as a hydrated form, several polymorphs of MnO2 are claimed. In the structure of the rutile crystal (this polymorph is called pyrolusite or β-MnO2), MnO2 crystallizes with three-coordinate oxide and octahedral metal centers, like several other dioxides. A significant component in batteries is manganese dioxide. It is also used for the removal of the green fin caused by iron impurities in glassmaking. MnO2 is characteristically nonstoichiometric, being oxygen deficient. This material’s complex solid-state chemistry is important in organic synthesis to the lore of “freshly prepared” MnO2.
In manufacturing glass, manganese dioxide is often used to bleach the color of any iron present. A pink coloration can be created in the glass if excess manganese dioxide is added, and it will appear black if a large excess is present. Manganese dioxide that occurs naturally contains impurities and a large amount of oxide of manganese(III). The γ purity modification sufficient for the battery industry is found in only a small number of deposits. Other applications for manganese dioxide include its use in organic chemistry as an oxidizing agent and in safety matches, fireworks, and flares as an oxidizing agent. It is also an effective adsorbent for sulphide hydrogen and is also used to paint ceramics.
MnO2‘s major reactions are related to its redox, both oxidation, and reduction. Manganese metal, most manganese salts, and even manganese steel and other alloys are made using the mineral. MnO2 catalyzes many O2-forming reactions. The heating of a mixture of potassium chlorate and manganese dioxide creates oxygen gas in a classical laboratory demonstration. The decomposition of hydrogen peroxide into oxygen and water is also catalyzed by manganese dioxide:
2 H2O2 → 2 H2O + O2
The oxide is also a brick, tile, porcelain, and glass colorant; a paint and varnish drier; a 552 MANGANESE(IV) OXIDE preparation for textile printing and dyeing; a polysulfide rubber curing agent; an adsorbent for hydrogen sulfide and sulfur dioxide; an oxidizing agent in many organic syntheses such as quinone and hydroquinone; and a catalyst in the laboratory preparation of oxygen from potassium chlorate. Manganese dioxide decomposes into manganese(III) oxide and oxygen above around 530°C. The mixed-valence compound Mn3O4 forms at temperatures close to 1000°C. Man is given by higher temperatures.
Information Sources: